Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction
- PMID: 35053023
- PMCID: PMC8772848
- DOI: 10.3390/biology11010026
Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction
Abstract
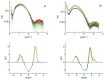
The early impairments appearing in Alzheimer's disease are related to neuronal membrane damage. Both aberrant Aβ species and specific membrane components play a role in promoting aggregation, deposition, and signaling dysfunction. Ganglioside GM1, present with cholesterol and sphingomyelin in lipid rafts, preferentially interacts with the Aβ peptide. GM1 at physiological conditions clusters in the membrane, the assembly also involves phospholipids, sphingomyelin, and cholesterol. The structure of large unilamellar vesicles (LUV), made of a basic POPC:POPS matrix in a proportion of 9:1, and containing different amounts of GM1 (1%, 3%, and 4% mol/mol) in the presence of 5% mol/mol sphingomyelin and 15% mol/mol cholesterol, was studied using small angle X-ray scattering (SAXS). The effect of the membrane composition on the LUVs-Aβ-peptide interaction, both for Aβ1-40 and Aβ1-42 variants, was, thus, monitored. The presence of GM1 leads to a significant shift of the main peak, towards lower scattering angles, up to 6% of the initial value with SM and 8% without, accompanied by an opposite shift of the first minimum, up to 21% and 24% of the initial value, respectively. The analysis of the SAXS spectra, using a multi-Gaussian model for the electronic density profile, indicated differences in the bilayer of the various compositions. An increase in the membrane thickness, by 16% and 12% when 2% and 3% mol/mol GM1 was present, without and with SM, respectively, was obtained. Furthermore, in these cases, in the presence of Aβ40, a very small decrease of the bilayer thickness, less than 4% and 1%, respectively, was derived, suggesting the inhibiting effect that the presence of sphingomyelin has on the GM1-Aβ interaction.
Keywords: Aβ; GM1; SAXS; large unilamellar vesicles; rafts; sphingomyelin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. - DOI - PMC - PubMed
-
- de Felice F.G., Wu D., Lambert M.P., Fernandez S.J., Velasco P.T., Lacor P.N., Bigio E.H., Jerecic J., Acton P.J., Shughrue P.J., et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. - DOI - PMC - PubMed
-
- Wirths O., Multhaup G., Czech C., Blanchard V., Moussaoui S., Tremp G., Pradier L., Beyreuther K., A Bayer T. Intraneuronal Aβ accumulation precedes plaque formation in β-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 2001;306:116–120. doi: 10.1016/S0304-3940(01)01876-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources

