Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds
- PMID: 35053239
- PMCID: PMC8774257
- DOI: 10.3390/biom12010090
Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds
Abstract
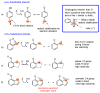
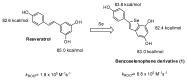
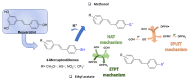
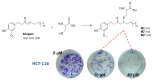
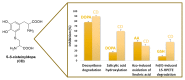
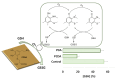
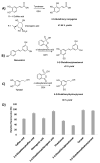
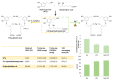
The activity of natural phenols is primarily associated to their antioxidant potential, but is ultimately expressed in a variety of biological effects. Molecular scaffold manipulation of this large variety of compounds is a currently pursued approach to boost or modulate their properties. Insertion of S/Se/Te containing substituents on phenols may increase/decrease their H-donor/acceptor ability by electronic and stereo-electronic effects related to the site of substitution and geometrical constrains. Oxygen to sulphur/selenium isosteric replacement in resveratrol or ferulic acid leads to an increase in the radical scavenging activity with respect to the parent phenol. Several chalcogen-substituted phenols inspired by Vitamin E and flavonoids have been prepared, which in some cases prove to be chain-breaking antioxidants, far better than the natural counterparts. Conjugation of catechols with biological thiols (cysteine, glutathione, dihydrolipoic acid) is easily achieved by addition to the corresponding ortho-quinones. Noticeable examples of compounds with potentiated antioxidant activities are the human metabolite 5-S-cysteinyldopa, with high iron-induced lipid peroxidation inhibitory activity, due to strong iron (III) binding, 5-S-glutathionylpiceatannol a most effective inhibitor of nitrosation processes, and 5-S-lipoylhydroxytyrosol, and its polysulfides that proved valuable oxidative-stress protective agents in various cellular models. Different methodologies have been used for evaluation of the antioxidant power of these compounds against the parent compounds. These include kinetics of inhibition of lipid peroxidation alkylperoxyl radicals, common chemical assays of radical scavenging, inhibition of the OH• mediated hydroxylation/oxidation of model systems, ferric- or copper-reducing power, scavenging of nitrosating species. In addition, computational methods allowed researchers to determine the Bond Dissociation Enthalpy values of the OH groups of chalcogen modified phenolics and predict the best performing derivative. Finally, the activity of Se and Te containing compounds as mimic of glutathione peroxidase has been evaluated, together with other biological activities including anticancer action and (neuro)protective effects in various cellular models. These and other achievements are discussed and rationalized to guide future development in the field.
Keywords: Bond Dissociation Energy; DFT calculations; S/Se/Te-substituted phenols; alkylperoxyl radicals; antioxidant chemical assays; dihydrolipoic acid; glutathione peroxidase-like activity; glutathionyl/cysteinylcatechols; kinetic constant; lipid peroxidation; thia/selenotocopherols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Olivares-Vicente M., Barrajon-Catalan E., Herranz-Lopez M., Segura-Carretero A., Joven J., Encinar J.A., Micol V. Plant-Derived Polyphenols in Human Health: Biological Activity, Metabolites and Putative Molecular Targets. Curr. Drug Metabol. 2018;19:351–369. doi: 10.2174/1389200219666180220095236. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

