Comparison of Antiplatelet Effects of Phenol Derivatives in Humans
- PMID: 35053265
- PMCID: PMC8774223
- DOI: 10.3390/biom12010117
Comparison of Antiplatelet Effects of Phenol Derivatives in Humans
Abstract
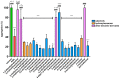
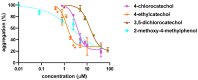
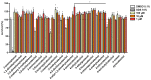
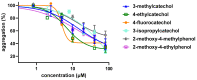
Flavonoids are associated with positive cardiovascular effects. However, due to their low bioavailability, metabolites are likely responsible for these properties. Recently, one of these metabolites, 4-methylcatechol, was described to be a very potent antiplatelet compound. This study aimed to compare its activity with its 22 close derivatives both of natural or synthetic origin in order to elucidate a potential structure-antiplatelet activity relationship. Blood from human volunteers was induced to aggregate by arachidonic acid (AA), collagen or thrombin, and plasma coagulation was also studied. Potential toxicity was tested on human erythrocytes as well as on a cancer cell line. Our results indicated that 17 out of the 22 compounds were very active at a concentration of 40 μM and, importantly, seven of them had an IC50 on AA-triggered aggregation below 3 μM. The effects of the most active compounds were confirmed on collagen-triggered aggregation too. None of the tested compounds was toxic toward erythrocytes at 50 μM and four compounds partly inhibited proliferation of breast cancer cell line at 100 μM but not at 10 μM. Additionally, none of the compounds had a significant effect on blood coagulation or thrombin-triggered aggregation. This study hence reports four phenol derivatives (4-ethylcatechol, 4-fluorocatechol, 2-methoxy-4-ethylphenol and 3-methylcatechol) suitable for future in vivo testing.
Keywords: 4-methylcatechol; aggregation; catechol; flavonoid; platelet; whole blood.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
4-Methylcatechol, a Flavonoid Metabolite with Potent Antiplatelet Effects.Mol Nutr Food Res. 2019 Aug 7;63(20):e1900261. doi: 10.1002/mnfr.201900261. Mol Nutr Food Res. 2019. PMID: 31343835
-
New platelet aggregation inhibitors based on pyridazinone moiety.Eur J Med Chem. 2015 Apr 13;94:113-22. doi: 10.1016/j.ejmech.2015.02.061. Epub 2015 Mar 3. Eur J Med Chem. 2015. PMID: 25757094
-
Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition.Eur J Pharmacol. 2010 Jul 25;638(1-3):5-12. doi: 10.1016/j.ejphar.2010.04.003. Epub 2010 Apr 20. Eur J Pharmacol. 2010. PMID: 20412790
-
In vitro antiplatelet activity of flavonoids from Leuzea carthamoides.Drug Chem Toxicol. 2008;31(1):27-35. doi: 10.1080/01480540701688444. Drug Chem Toxicol. 2008. PMID: 18161506
-
Inhibitory effects of phenol derivatives on bovine platelet aggregation and their effects on Ca2+ mobilization.Biochim Biophys Acta. 1989 Mar 28;1011(1):52-7. doi: 10.1016/0167-4889(89)90077-3. Biochim Biophys Acta. 1989. PMID: 2493810
Cited by
-
The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action.Nutrients. 2022 Nov 13;14(22):4798. doi: 10.3390/nu14224798. Nutrients. 2022. PMID: 36432485 Free PMC article.
-
Acetylsalicylic acid and vorapaxar are less active, while 4-methylcatechol is more active, in type 1 diabetic patients compared to healthy controls.Cardiovasc Diabetol. 2025 Aug 7;24(1):323. doi: 10.1186/s12933-025-02891-6. Cardiovasc Diabetol. 2025. PMID: 40775339 Free PMC article.
-
Small phenolic compounds as potential endocrine disruptors interacting with estrogen receptor alpha.Front Endocrinol (Lausanne). 2024 Oct 24;15:1440654. doi: 10.3389/fendo.2024.1440654. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39512757 Free PMC article.
References
-
- Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. - DOI - PMC - PubMed
-
- Kokubo Y., Iso H., Ishihara J., Okada K., Inoue M., Tsugane S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. - DOI - PubMed
-
- Mursu J., Voutilainen S., Nurmi T., Tuomainen T.P., Kurl S., Salonen J.T. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2008;100:890–895. doi: 10.1017/S0007114508945694. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

