Synthesis and Biological Evaluation of Benzo[ b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus
- PMID: 35053281
- PMCID: PMC8773820
- DOI: 10.3390/biom12010131
Synthesis and Biological Evaluation of Benzo[ b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus
Abstract
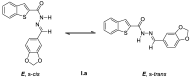
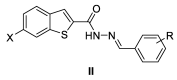
The benzo[b]thiophene nucleus and the acylhydrazone functional group were combined to prepare three new series of compounds for screening against Staphylococcus aureus. The reaction of substituted benzo[b]thiophene-2-carboxylic hydrazide and various aromatic or heteroaromatic aldehydes led to a collection of 26 final products with extensive structural diversification on the aromatic ring and on position 6 of the benzo[b]thiophene nucleus. The screening lead to the identification of eight hits, including (E)-6-chloro-N'-(pyridin-2-ylmethylene)benzo[b]thiophene-2-carbohydrazide (II.b), a non-cytotoxic derivative showing a minimal inhibitory concentration of 4 µg/mL on three S. aureus strains, among which were a reference classical strain and two clinically isolated strains resistant to methicillin and daptomycin, respectively.
Keywords: MRSA; Staphylococcus aureus; acylhydrazone; antibacterial; benzothiophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Bradley J.S., Guidos R., Baragona S., Bartlett J.G., Rubinstein E., Zhanel G.G., Tino M.D., Pompliano D.L., Tally F., Tipirneni P., et al. Anti-infective research and development--problems, challenges, and solutions. Lancet Infect. Dis. 2007;7:68–78. doi: 10.1016/S1473-3099(06)70689-2. - DOI - PubMed
-
- Neill J.O. Review Paper-Tackling a Crisis for the Health and Wealth of Nations. HM Government Wellcome Trust; London, UK: 2014. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations; pp. 1–20.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

