Accelerated Aging Characterizes the Early Stage of Alzheimer's Disease
- PMID: 35053352
- PMCID: PMC8774248
- DOI: 10.3390/cells11020238
Accelerated Aging Characterizes the Early Stage of Alzheimer's Disease
Abstract
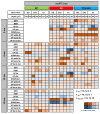
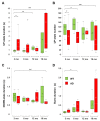
For Alzheimer's disease (AD), aging is the main risk factor, but whether cognitive impairments due to aging resemble early AD deficits is not yet defined. When working with mouse models of AD, the situation is just as complicated, because only a few studies track the progression of the disease at different ages, and most ignore how the aging process affects control mice. In this work, we addressed this problem by comparing the aging process of PS2APP (AD) and wild-type (WT) mice at the level of spontaneous brain electrical activity under anesthesia. Using local field potential recordings, obtained with a linear probe that traverses the posterior parietal cortex and the entire hippocampus, we analyzed how multiple electrical parameters are modified by aging in AD and WT mice. With this approach, we highlighted AD specific features that appear in young AD mice prior to plaque deposition or that are delayed at 12 and 16 months of age. Furthermore, we identified aging characteristics present in WT mice but also occurring prematurely in young AD mice. In short, we found that reduction in the relative power of slow oscillations (SO) and Low/High power imbalance are linked to an AD phenotype at its onset. The loss of SO connectivity and cortico-hippocampal coupling between SO and higher frequencies as well as the increase in UP-state and burst durations are found in young AD and old WT mice. We show evidence that the aging process is accelerated by the mutant PS2 itself and discuss such changes in relation to amyloidosis and gliosis.
Keywords: Alzheimer’s disease; PS2APP; UP-DOWN states; amyloid-β; delta waves; functional connectivity; phase-amplitude-coupling; presenilin-2; slow oscillations; spikes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jelic V., Johansson S.E., Almkvist O., Shigeta M., Julin P., Nordberg A., Winblad B., Wahlund L.O. Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. - DOI - PubMed
-
- Prichep L.S., John E.R., Ferris S.H., Rausch L., Fang Z., Cancro R., Torossian C., Reisberg B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol. Aging. 2006;27:471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

