Mesenchymal Stem Cell Therapy in Diabetic Cardiomyopathy
- PMID: 35053356
- PMCID: PMC8773977
- DOI: 10.3390/cells11020240
Mesenchymal Stem Cell Therapy in Diabetic Cardiomyopathy
Abstract
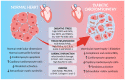
The incidence and prevalence of diabetes mellitus (DM) are increasing worldwide, and the resulting cardiac complications are the leading cause of death. Among these complications is diabetes-induced cardiomyopathy (DCM), which is the consequence of a pro-inflammatory condition, oxidative stress and fibrosis caused by hyperglycemia. Cardiac remodeling will lead to an imbalance in cell survival and death, which can promote cardiac dysfunction. Since the conventional treatment of DM generally does not address the prevention of cardiac remodeling, it is important to develop new alternatives for the treatment of cardiovascular complications induced by DM. Thus, therapy with mesenchymal stem cells has been shown to be a promising approach for the prevention of DCM because of their anti-apoptotic, anti-fibrotic and anti-inflammatory effects, which could improve cardiac function in patients with DM.
Keywords: cardiac remodeling; diabetes mellitus; diabetic cardiomyopathy; fibrosis; mesenchymal stromal cells; oxidative stress; pro-inflammatory cytokines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sinclair A., Saeedi P., Kaundal A., Karuranga S., Malanda B., Williams R. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020;162:108078. doi: 10.1016/j.diabres.2020.108078. - DOI - PubMed
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

