Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex
- PMID: 35053360
- PMCID: PMC8774241
- DOI: 10.3390/cells11020244
Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex
Abstract
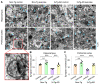
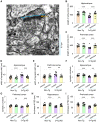
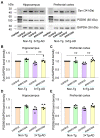
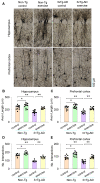
Alzheimer's disease (AD) is characterized by deficits in learning and memory. A pathological feature of AD is the alterations in the number and size of synapses, axon length, dendritic complexity, and dendritic spine numbers in the hippocampus and prefrontal cortex. Treadmill exercise can enhance synaptic plasticity in mouse or rat models of stroke, ischemia, and dementia. The aim of this study was to examine the effects of treadmill exercise on learning and memory, and structural synaptic plasticity in 3×Tg-AD mice, a mouse model of AD. Here, we show that 12 weeks treadmill exercise beginning in three-month-old mice improves spatial working memory in six-month-old 3×Tg-AD mice, while non-exercise six-month-old 3×Tg-AD mice exhibited impaired spatial working memory. To investigate potential mechanisms for the treadmill exercise-induced improvement of spatial learning and memory, we examined structural synaptic plasticity in the hippocampus and prefrontal cortex of six-month-old 3×Tg-AD mice that had undergone 12 weeks of treadmill exercise. We found that treadmill exercise led to increases in synapse numbers, synaptic structural parameters, the expression of synaptophysin (Syn, a presynaptic marker), the axon length, dendritic complexity, and the number of dendritic spines in 3×Tg-AD mice and restored these parameters to similar levels of non-Tg control mice without treadmill exercise. In addition, treadmill exercise also improved these parameters in non-Tg control mice. Strengthening structural synaptic plasticity may represent a potential mechanism by which treadmill exercise prevents decline in spatial learning and memory and synapse loss in 3×Tg-AD mice.
Keywords: 3×Tg-AD mice; dendritic spines; exercise; memory; structural synaptic plasticity; synaptic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

