Glucose Regulates m6A Methylation of RNA in Pancreatic Islets
- PMID: 35053407
- PMCID: PMC8773766
- DOI: 10.3390/cells11020291
Glucose Regulates m6A Methylation of RNA in Pancreatic Islets
Abstract
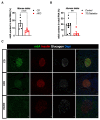
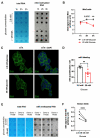
Type 2 diabetes is characterized by chronic hyperglycemia associated with impaired insulin action and secretion. Although the heritability of type 2 diabetes is high, the environment, including blood components, could play a major role in the development of the disease. Amongst environmental effects, epitranscriptomic modifications have been recently shown to affect gene expression and glucose homeostasis. The epitranscriptome is characterized by reversible chemical changes in RNA, with one of the most prevalent being the m6A methylation of RNA. Since pancreatic β cells fine tune glucose levels and play a major role in type 2 diabetes physiopathology, we hypothesized that the environment, through variations in blood glucose or blood free fatty acid concentrations, could induce changes in m6A methylation of RNAs in pancreatic β cells. Here we observe a significant decrease in m6A methylation upon high glucose concentration, both in mice and human islets, associated with altered expression levels of m6A demethylases. In addition, the use of siRNA and/or specific inhibitors against selected m6A enzymes demonstrate that these enzymes modulate the expression of genes involved in pancreatic β-cell identity and glucose-stimulated insulin secretion. Our data suggest that environmental variations, such as glucose, control m6A methylation in pancreatic β cells, playing a key role in the control of gene expression and pancreatic β-cell functions. Our results highlight novel causes and new mechanisms potentially involved in type 2 diabetes physiopathology and may contribute to a better understanding of the etiology of this disease.
Keywords: epitranscriptome; insulin secretion; pancreatic beta cell; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ndiaye F.K., Ortalli A., Canouil M., Huyvaert M., Salazar-Cardozo C., Lecoeur C., Verbanck M., Pawlowski V., Boutry R., Durand E., et al. Expression and Functional Assessment of Candidate Type 2 Diabetes Susceptibility Genes Identify Four New Genes Contributing to Human Insulin Secretion. Mol. Metab. 2017;6:459–470. doi: 10.1016/j.molmet.2017.03.011. - DOI - PMC - PubMed
-
- Ohn J.H., Kwak S.H., Cho Y.M., Lim S., Jang H.C., Park K.S., Cho N.H. 10-Year Trajectory of β-Cell Function and Insulin Sensitivity in the Development of Type 2 Diabetes: A Community-Based Prospective Cohort Study. Lancet Diabetes Endocrinol. 2016;4:27–34. doi: 10.1016/S2213-8587(15)00336-8. - DOI - PubMed
-
- Levy J., Atkinson A.B., Bell P.M., McCance D.R., Hadden D.R. Beta-Cell Deterioration Determines the Onset and Rate of Progression of Secondary Dietary Failure in Type 2 Diabetes Mellitus: The 10-Year Follow-up of the Belfast Diet Study. Diabet. Med. 1998;15:290–296. doi: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

