Pathophysiological Integration of Metabolic Reprogramming in Breast Cancer
- PMID: 35053485
- PMCID: PMC8773662
- DOI: 10.3390/cancers14020322
Pathophysiological Integration of Metabolic Reprogramming in Breast Cancer
Abstract
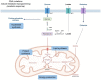
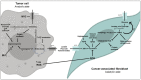
Metabolic changes that facilitate tumor growth are one of the hallmarks of cancer. The triggers of these metabolic changes are located in the tumor parenchymal cells, where oncogenic mutations induce an imperative need to proliferate and cause tumor initiation and progression. Cancer cells undergo significant metabolic reorganization during disease progression that is tailored to their energy demands and fluctuating environmental conditions. Oxidative stress plays an essential role as a trigger under such conditions. These metabolic changes are the consequence of the interaction between tumor cells and stromal myofibroblasts. The metabolic changes in tumor cells include protein anabolism and the synthesis of cell membranes and nucleic acids, which all facilitate cell proliferation. They are linked to catabolism and autophagy in stromal myofibroblasts, causing the release of nutrients for the cells of the tumor parenchyma. Metabolic changes lead to an interstitium deficient in nutrients, such as glucose and amino acids, and acidification by lactic acid. Together with hypoxia, they produce functional changes in other cells of the tumor stroma, such as many immune subpopulations and endothelial cells, which lead to tumor growth. Thus, immune cells favor tissue growth through changes in immunosuppression. This review considers some of the metabolic changes described in breast cancer.
Keywords: cancer-associated fibroblasts; glucose; hypoxia; interstitium; lactate; macrophages; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A.K., Frank P.G., Casimiro M.C., Wang C., Fortina P., Addya S., et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. - DOI - PubMed
-
- Wilde L., Roche M., Domingo-Vidal M., Tanson K., Philp N., Curry J., Martinez-Outschoorn U. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. - DOI - PMC - PubMed
Publication types
Grants and funding
- PID2020-118527RB-I00/Ministerio de Ciencia e Innovación
- PDC2021-121735-I00/Ministerio de Ciencia e Innovación
- PIE14/00066/Carlos III Health Institute
- CSI234P18/Regional Government of Castile and León
- CSI144P20/Regional Government of Castile and León
- RTI2018-094130-B-100/Ministerio de Ciencia e Innovación
- SAF2014-56989-R/Ministerio de Economía, Industria y Competitividad
- SAF2017-88854R/Ministerio de Economía, Industria y Competitividad
- GRS2139/A/20/Gerencia Regional de Salud de Castilla y León
- PI18/00587/Instituto de Salud Carlos III
- PI21/01207/Instituto de Salud Carlos III

