Decellularized Colorectal Cancer Matrices as Bioactive Scaffolds for Studying Tumor-Stroma Interactions
- PMID: 35053521
- PMCID: PMC8773780
- DOI: 10.3390/cancers14020359
Decellularized Colorectal Cancer Matrices as Bioactive Scaffolds for Studying Tumor-Stroma Interactions
Abstract
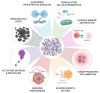
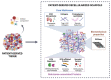
More than a physical structure providing support to tissues, the extracellular matrix (ECM) is a complex and dynamic network of macromolecules that modulates the behavior of both cancer cells and associated stromal cells of the tumor microenvironment (TME). Over the last few years, several efforts have been made to develop new models that accurately mimic the interconnections within the TME and specifically the biomechanical and biomolecular complexity of the tumor ECM. Particularly in colorectal cancer, the ECM is highly remodeled and disorganized and constitutes a key component that affects cancer hallmarks, such as cell differentiation, proliferation, angiogenesis, invasion and metastasis. Therefore, several scaffolds produced from natural and/or synthetic polymers and ceramics have been used in 3D biomimetic strategies for colorectal cancer research. Nevertheless, decellularized ECM from colorectal tumors is a unique model that offers the maintenance of native ECM architecture and molecular composition. This review will focus on innovative and advanced 3D-based models of decellularized ECM as high-throughput strategies in colorectal cancer research that potentially fill some of the gaps between in vitro 2D and in vivo models. Our aim is to highlight the need for strategies that accurately mimic the TME for precision medicine and for studying the pathophysiology of the disease.
Keywords: colorectal cancer; extracellular matrix; patient-derived scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

