Psychometric Validation of the German Translation of the Quality of Life Questionnaire-Bronchiectasis (QOL-B)-Data from the German Bronchiectasis Registry PROGNOSIS
- PMID: 35054135
- PMCID: PMC8781204
- DOI: 10.3390/jcm11020441
Psychometric Validation of the German Translation of the Quality of Life Questionnaire-Bronchiectasis (QOL-B)-Data from the German Bronchiectasis Registry PROGNOSIS
Abstract
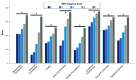
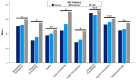
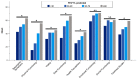
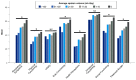
Patients with bronchiectasis feature considerable symptom burden and reduced health-related quality of life (QOL). We provide the psychometric validation of the German translation of the disease-specific Quality of Life Questionnaire-Bronchiectasis (QOL-B), version 3.1, using baseline data of adults consecutively enrolled into the prospective German bronchiectasis registry PROGNOSIS. Overall, 904 patients with evaluable QOL-B scores were included. We observed no relevant floor or ceiling effects. Internal consistency was good to excellent (Cronbach's α ≥0.73 for each scale). QOL-B scales discriminated between patients based on prior pulmonary exacerbations and hospitalizations, breathlessness, bronchiectasis severity index, lung function, sputum volume, Pseudomonas aeruginosa status and the need for regular pharmacotherapy, except for Social Functioning, Vitality and Emotional Functioning scales. We observed moderate to strong convergence between several measures of disease severity and QOL-B scales, except for Social and Emotional Functioning. Two-week test-retest reliability was good, with intraclass correlation coefficients ≥0.84 for each scale. Minimal clinical important difference ranged between 8.5 for the Respiratory Symptoms and 14.1 points for the Social Functioning scale. Overall, the German translation of the QOL-B, version 3.1, has good validity and test-retest reliability among a nationally representative adult bronchiectasis cohort. However, responsiveness of QOL-B scales require further investigation during registry follow-up.
Keywords: Germany; bronchiectasis; patient-reported outcome measures; quality of life; questionnaire design; registries.
Conflict of interest statement
L.Q., G.B.N., A.D.R. and A.Z. declare no conflicts of interest. R.D. and/or his institution received grants, advisory/lecture/clinical trial fees and non-financial support by Bayer, Cepheid, DiaSorin, Insmed, Oxford Immunotec, Qiagen, Quidel, BMBF, DZL and DZIF. P.M. and/or his institution received grants, advisory/lecture/clinical trial fees and non-financial support by AstraZeneca, Boehringer Ingelheim, Insmed Inc., Vertex and DZL. I.P. and/or her institution received grants, advisory/lecture/clinical trial fees and non-financial support from Chiesi and Förderstiftung MHH Plus. J.R. and/or her institution received grants, advisory/lecture/clinical trial fees and non-financial support from AstraZeneca, Bayer, Brahms, Chiesi, Esanum, Grifols, GSK, Novartis, DZL, DZIF and BMBF. S.S. received personal fees or grants from Boehringer Ingelheim, Celtaxsys, Chiesi, Corbus Pharmaceuticals Flatley, Galapagos, Insmed Inc., Ionis Pharmaceuticals, Novartis, Proteostasis Therapeutics, Teva and Vertex outside the submitted work. S.S. has served as an investigator in clinical trials for AstraZeneca, Zambon, Insmed Inc., Novartis and Vertex. T.W. received grants to his institution from Insmed Inc. and Novartis and personal fees for advisory boards and lectures by AstraZeneca, Insmed Inc., Novartis and Zambon. F.C.R. and/or his institution received grants, advisory/lecture/clinical trial fees and non-financial support by AstraZeneca, Bayer, Boehringer Ingelheim, Celtaxsys, Chiesi, Corbus, Grifols, InfectoPharm, Insmed Inc., Novartis, PARI, Parion, Polyphor, Shionogi, Vertex, Zambon, DZL, DZIF, BMBF and IMI/EFPIA.
Figures
References
-
- Aliberti S., Goeminne P.C., O’Donnell A.E., Aksamit T.R., Al-Jahdali H., Barker A.F., Blasi F., Boersma W.G., Crichton M.L., De Soyza A., et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: International consensus recommendations. Lancet Respir. Med. 2021. Online ahead of print . - PubMed
-
- Quint J.K., Millett E.R., Joshi M., Navaratnam V., Thomas S.L., Hurst J.R., Smeeth L., Brown J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. - DOI - PMC - PubMed
-
- Loebinger M.R., Polverino E., Chalmers J.D., Tiddens H., Goossens H., Tunney M., Ringshausen F.C., Hill A.T., Pathan R., Angyalosi G., et al. Efficacy and safety of TOBI podhaler in pseudomonas aeruginosa-infected bronchiectasis patients: iBEST study. Eur. Respir. J. 2021;57:2001451. doi: 10.1183/13993003.01451-2020. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical

