Lymphatic Endothelial Markers and Tumor Lymphangiogenesis Assessment in Human Breast Cancer
- PMID: 35054174
- PMCID: PMC8774380
- DOI: 10.3390/diagnostics12010004
Lymphatic Endothelial Markers and Tumor Lymphangiogenesis Assessment in Human Breast Cancer
Abstract
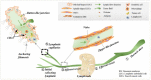
Metastasis via lymphatic vessels or blood vessels is the leading cause of death for breast cancer, and lymphangiogenesis and angiogenesis are critical prerequisites for the tumor invasion-metastasis cascade. The research progress for tumor lymphangiogenesis has tended to lag behind that for angiogenesis due to the lack of specific markers. With the discovery of lymphatic endothelial cell (LEC) markers, growing evidence demonstrates that the LEC plays an active role in lymphatic formation and remodeling, tumor cell growth, invasion and intravasation, tumor-microenvironment remodeling, and antitumor immunity. However, some studies have drawn controversial conclusions due to the variation in the LEC markers and lymphangiogenesis assessments used. In this study, we review recent findings on tumor lymphangiogenesis, the most commonly used LEC markers, and parameters for lymphangiogenesis assessments, such as the lymphatic vessel density and lymphatic vessel invasion in human breast cancer. An in-depth understanding of tumor lymphangiogenesis and LEC markers can help to illustrate the mechanisms and distinct roles of lymphangiogenesis in breast cancer progression, which will help in exploring novel potential predictive biomarkers and therapeutic targets for breast cancer.
Keywords: breast cancer; lymphangiogenesis; lymphatic endothelial cell; lymphatic vessel density; lymphatic vessel invasion; markers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020. [(accessed on 15 December 2020)]. Available online: https://www.iarc.fr/wp-content/uploads/2020/12/pr292_E.pdf.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

