Assessing SARS-CoV-2 Neutralizing Antibodies after BNT162b2 Vaccination and Their Correlation with SARS-CoV-2 IgG Anti-S1, Anti-RBD and Anti-S2 Serological Titers
- PMID: 35054372
- PMCID: PMC8775066
- DOI: 10.3390/diagnostics12010205
Assessing SARS-CoV-2 Neutralizing Antibodies after BNT162b2 Vaccination and Their Correlation with SARS-CoV-2 IgG Anti-S1, Anti-RBD and Anti-S2 Serological Titers
Abstract
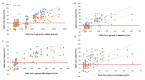
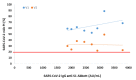
The humoral response through neutralizing antibodies (NAbs) is a key component of the immune response to COVID-19. However, the plaque reduction neutralization test (PRNT), the gold standard for determining NAbs, is technically demanding, time-consuming and requires BSL-3 conditions. Correlating the NAbs and total antibodies levels, assessed by generalized and automated serological tests, is crucial. Through a commercial surrogate virus neutralization test (sVNT), we aimed to evaluate the production of SARS-CoV-2 NAbs in a set of vaccinated healthcare workers and to correlate these NAbs with the SARS-CoV-2 IgG anti-S1, anti-RBD and anti-S2 serological titers. We found that 6 months after vaccination, only 74% maintain NAbs for the Wuhan strain/UK variant (V1) and 47% maintain NAbs for the South African and Brazil variants (V2). Through Spearman's correlation, we found the following correlations between the percentage of inhibition of NAbs and the SARS-CoV-2 IgG II Quant (Abbott Laboratories, Chicago, IL, USA) and BioPlex 2200 SARS-CoV-2 IgG Panel (Bio-Rad, Hercules, CA, USA) immunoassays: rho = 0.87 (V1) and rho = 0.73 (V2) for anti-S1 assessed by Abbott assay; rho = 0.77 (V1) and rho = 0.72 (V2) for anti-S1, rho = 0.88 (V1) and rho = 0.82 (V2) for anti-RBD, and rho = 0.68 (V1) and rho = 0.60 (V2) for anti-S2 assessed by BioPlex assay (p < 0.001 for all). In conclusion, we found a strong correlation between this fast, user-friendly, mobile and bio-safe sVNT and the serological immunoassays.
Keywords: BNT162b2 vaccine; SARS-CoV-2; neutralizing antibodies; serological immunoassays; surrogate virus neutralization test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., Wellington E., Stowe J., Gillson N., Atti A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. - DOI - PMC - PubMed
-
- Jalkanen P., Kolehmainen P., Häkkinen H.K., Huttunen M., Tähtinen P.A., Lundberg R., Maljanen S., Reinholm A., Tauriainen S., Pakkanen S.H., et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021;12:3991. doi: 10.1038/s41467-021-24285-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

