Preclinical Validation of a Novel Injection-Molded Swab for the Molecular Assay Detection of SARS-CoV-2
- PMID: 35054373
- PMCID: PMC8775180
- DOI: 10.3390/diagnostics12010206
Preclinical Validation of a Novel Injection-Molded Swab for the Molecular Assay Detection of SARS-CoV-2
Abstract
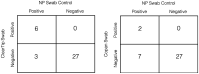
During the COVID-19 public health emergency, many actions have been undertaken to help ensure that patients and health care providers have timely and continued access to high-quality medical devices to respond effectively. The development and validation of new testing supplies and equipment, including collection swabs, has helped to expand the availability and capability for various diagnostic, therapeutic, and protective medical devices in high demand during the COVID-19 emergency. Here, we report the initial validation of a new injection-molded anterior nasal swab, ClearTip™, that was experimentally validated in a laboratory setting as well as in independent clinical studies in comparison to gold standard flocked swabs. We have also developed an in vitro anterior nasal tissue model which offers a novel, efficient, and clinically relevant validation tool to replicate the clinical swabbing workflow with high fidelity, while being accessible, safe, reproducible, and time- and cost-effective. ClearTip™ displayed greater inactivated virus release in the benchtop model, confirmed by its greater ability to report positive samples in a small clinical study in comparison to flocked swabs. We also quantified the detection of biological materials, as a proxy for viral material, in multi-center pre-clinical and clinical studies which showed a statistically significant difference in one study and a reduction in performance in comparison to flocked swabs. Taken together, these results emphasize the compelling benefits of non-absorbent injection-molded anterior nasal swabs for COVID-19 detection, comparable to standard flocked swabs. Injection-molded swabs, as ClearTip™, could have the potential to support future swab shortages, due to its manufacturing advantages, while offering benefits in comparison to highly absorbent swabs in terms of comfort, limited volume collection, and potential multiple usage.
Keywords: COVID-19; SARS-CoV-2; diagnostic testing; swab; tissue model; virological testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Coronavirus Cases. [(accessed on 27 July 2021)]. Available online: https://www.worldometers.info/coronavirus/
-
- WHO Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. [(accessed on 27 July 2021)]. Interim Guidance. Available online: https://www.who.int/publications/i/item/10665-331501.
-
- Callahan C.J., Lee R., Zulauf K.E., Tamburello L., Smith K.P., Previtera J., Cheng A., Green A., Azim A.A., Yano A., et al. Open Development and Clinical Validation of Multiple 3D-Printed Nasopharyngeal Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck. J. Clin. Microbiol. 2020;58:e00876-20. doi: 10.1128/JCM.00876-20. - DOI - PMC - PubMed
-
- Moisset X., Gautier N., Godet T., Parabère S., Pereira B., Meunier E., Gerbaud L., Lesens O., Henquell C., Beytout J., et al. Nasopharyngeal swab-induced pain for SARS-CoV-2 screening: A randomised controlled trial of conventional and self-swabbing. Eur. J. Pain. 2021;25:924–929. doi: 10.1002/ejp.1722. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

