Loss of Corticostriatal Mu-Opioid Receptors in α-Synuclein Transgenic Mouse Brains
- PMID: 35054456
- PMCID: PMC8781165
- DOI: 10.3390/life12010063
Loss of Corticostriatal Mu-Opioid Receptors in α-Synuclein Transgenic Mouse Brains
Abstract
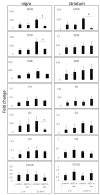
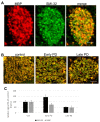
Ultrastructural, neurochemical, and molecular alterations within the striatum are associated with the onset and progression of Parkinson's disease (PD). In PD, the dopamine-containing neurons in the substantia nigra pars compacta (SNc) degenerate and reduce dopamine-containing innervations to the striatum. The loss of striatal dopamine is associated with enhanced corticostriatal glutamatergic plasticity at the early stages of PD. However, with disease progression, the glutamatergic corticostriatal white matter tracts (WMTs) also degenerate. We analyzed the levels of Mu opioid receptors (MORs) in the corticostriatal WMTs, as a function of α-Synuclein (α-Syn) toxicity in transgenic mouse brains. Our data show an age-dependent loss of MOR expression levels in the striatum and specifically, within the caudal striatal WMTs in α-Syn tg mouse brains. The loss of MOR expression is associated with degeneration of the myelinated axons that are localized within the corticostriatal WMTs. In brains affected with late stages of PD, we detect evidence confirming the degeneration of myelinated axons within the corticostriatal WMTs. We conclude that loss of corticostriatal MOR expression is associated with degeneration of corticostriatal WMT in α-Syn tg mice, modeling PD.
Keywords: corticostriatal glutamatergic axons; mu-opioid receptors (MOR); white matter tracts (WMTs); α-Synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Huston J.P. In: Hand Book of Basal Ganglia Structure and Function. 2nd ed. Steiner H., Tseng K.Y., editors. Volume 24. Elsevier; Cambridge, MA, USA: 2016. pp. 3–32. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

