Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study
- PMID: 35054523
- PMCID: PMC8781376
- DOI: 10.3390/life12010131
Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study
Abstract
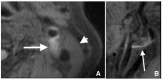
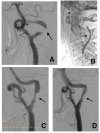
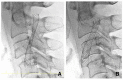
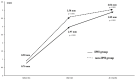
Vulnerable lesions with intraplaque hemorrhages are associated with a high incidence of complications following carotid artery stenting (CAS). CAS for vulnerable lesions has not been established; therefore, we gradually expand stents in such patients. This study aimed to compare the incidences of complications between gradual-expansion CAS for vulnerable lesions and standard CAS for non-vulnerable lesions. For gradual-expansion CAS, we used 3.0 or 4.0 mm balloons for minimal luminal diameters (MLDs) <2.0 or ≥2.0 mm, respectively, for pre-stenting angioplasty (SA) and did not overinflate them. By contrast, for standard CAS, we used a 4.0 mm balloon and overinflated it to 4.23 mm. A closed-cell stent was deployed, and post-SA was not performed in both groups. We evaluated the MLD before and minimal stent diameter (MSD) immediately after CAS, as well as periprocedural complications of combined stroke, death, and myocardial infarction within 30 days after CAS. In the vulnerable and non-vulnerable groups, 30 and 38 patients were analyzed, the MLDs were 0.76 and 0.96 mm before CAS, the MSDs were 2.97 mm and 3.58 mm after CAS, and the numbers of complications were 0 and 1, respectively. Gradual-expansion CAS for vulnerable lesions was as safe as standard CAS for non-vulnerable lesions.
Keywords: carotid artery stenting; intraplaque hemorrhage; periprocedural complications; post-stenting angioplasty; vulnerable lesion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Altaf N., Daniels L., Morgan P.S., Auer D., MacSweeney S.T., Moody A.R., Gladman J.R. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J. Vasc. Surg. 2008;47:337–342. doi: 10.1016/j.jvs.2007.09.064. - DOI - PubMed
-
- Saba L., Yuan C., Hatsukami T.S., Balu N., Qiao Y., DeMarco J.K., Saam T., Moody A.R., Li D., Matouk C.C., et al. Carotid artery wall imaging: Perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am. J. Neuroradiol. 2018;39:E9–E31. doi: 10.3174/ajnr.A5488. - DOI - PMC - PubMed
-
- Ito Y., Tsuruta W., Nakai Y., Takigawa T., Marushima A., Masumoto T., Matsumaru Y., Ishikawa E., Matsumura A. Treatment strategy based on plaque vulnerability and the treatment risk evaluation for internal carotid artery stenosis. Neurol. Med. Chir. 2018;58:191–198. doi: 10.2176/nmc.oa.2017-0228. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

