Synthesis and Excimer Formation Properties of Electroactive Polyamides Incorporated with 4,5-Diphenoxypyrene Units
- PMID: 35054668
- PMCID: PMC8778140
- DOI: 10.3390/polym14020261
Synthesis and Excimer Formation Properties of Electroactive Polyamides Incorporated with 4,5-Diphenoxypyrene Units
Abstract
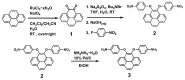
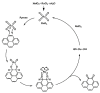
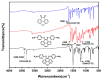
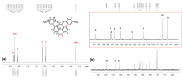
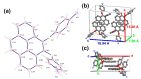
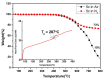
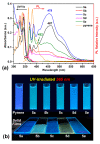
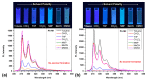
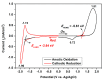
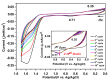
A new dietherpyrene-cored diamine monomer, namely, 4,5-bis(4-aminophenoxy)pyrene, was successful synthesized and formed a series of electroactive polyamides with an aryloxy linkage in a polymer main chain and bearing pyrene chromophore as a pendent group using conventional one-pot polycondensation reactions with commercial aromatic/aliphatic dicarboxylic acids. The resulting polyamides exhibited good solubility in polar organic solvents and, further, can be made into transparent films. They had appropriate levels of thermal stability with moderately high glass-transition values. The dilute NMP solutions of these polyamides exhibited pyrene characteristic fluorescence and also showed a remarkable additional excimer emission peak centered at 475 nm. Electrochemical studies of these polymer films showed that these polyamides have both p- and n-dopable states as a result of the formation of radical cations and anions of the electroactive pyrene moieties.
Keywords: electroactive polymers; excimer emission; high performance polymers; polyamides; pyrene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



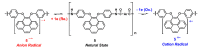


References
-
- Yang H.H. Aromatic High-Strength Fibers. John Wiley & Sons, Inc.; New York, NY, USA: 1989.
-
- Morgan P.W. Synthesis and properties of aromatic and extended chain polyamides. Macromolecules. 1977;10:1381–1390. doi: 10.1021/ma60060a040. - DOI
-
- Trigo-Lopez M., Estevez P., San-Jose N., Gomez-Valdemoro A., Garcia F.C., Serna F., de la Pena L.J., Garcia J.M. Recent Patents on Aromatic Polyamides. Recent Pat. Mater. Sci. 2009;2:190–208. doi: 10.2174/1874464810902030190. - DOI
-
- Cassidy P.E. Thermally Stable Polymers Syntheses and Properties. Marcel Dekker; New York, NY, USA: 1980.
-
- Dine-Hart R.A., Moore B.J., Wright W.W. Aromatic polyamides. J. Polym. Sci. Part B. 1964;2:369–373. doi: 10.1002/pol.1964.110020411. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

