Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-Up Approach-A Review
- PMID: 35054691
- PMCID: PMC8781687
- DOI: 10.3390/polym14020286
Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-Up Approach-A Review
Abstract
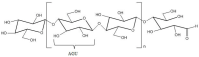
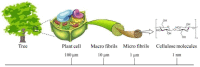
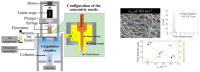
In the past decades, cellulose (one of the most important natural polymers), in the form of nanofibers, has received special attention. The nanofibrous morphology may provide exceptional properties to materials due to the high aspect ratio and dimensions in the nanometer range of the nanofibers. The first feature may lead to important consequences in mechanical behavior if there exists a particular orientation of fibers. On the other hand, nano-sizes provide a high surface-to-volume ratio, which can have important consequences on many properties, such as the wettability. There are two basic approaches for cellulose nanofibers preparation. The top-down approach implies the isolation/extraction of cellulose nanofibrils (CNFs) and nanocrystals (CNCs) from a variety of natural resources, whereby dimensions of isolates are limited by the source of cellulose and extraction procedures. The bottom-up approach can be considered in this context as the production of nanofibers using various spinning techniques, resulting in nonwoven mats or filaments. During the spinning, depending on the method and processing conditions, good control of the resulting nanofibers dimensions and, consequently, the properties of the produced materials, is possible. Pulp, cotton, and already isolated CNFs/CNCs may be used as precursors for spinning, alongside cellulose derivatives, namely esters and ethers. This review focuses on various spinning techniques to produce submicrometric fibers comprised of cellulose and cellulose derivatives. The spinning of cellulose requires the preparation of spinning solutions; therefore, an overview of various solvents is presented showing their influence on spinnability and resulting properties of nanofibers. In addition, it is shown how bottom-up spinning techniques can be used for recycling cellulose waste into new materials with added value. The application of produced cellulose fibers in various fields is also highlighted, ranging from drug delivery systems, high-strength nonwovens and filaments, filtration membranes, to biomedical scaffolds.
Keywords: cellulose; cellulose derivatives; nanofibers; spinning techniques.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Klemm D., Philipp B., Heinze T., Heinze U., Wagenknecht W. Comprehensive Cellulose Chemistry Volume l Fundamentals and Analytical Methods. Volume 1. Wiley-VCH; Weinheim, Germany: 1998.
-
- Ibrahim H.M., Klingner A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020;90:106647. doi: 10.1016/j.polymertesting.2020.106647. - DOI
-
- Thenmozhi S., Dharmaraj N., Kadirvelu K., Kim H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017;217:36–48. doi: 10.1016/j.mseb.2017.01.001. - DOI
-
- Barhoum A., Pal K., Rahier H., Uludag H., Kim I.S., Bechelany M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today. 2019;17:1–35. doi: 10.1016/j.apmt.2019.06.015. - DOI
-
- Bachs-Herrera A., Yousefzade O., Del Valle L.J., Puiggali J. Melt electrospinning of polymers: Blends, nanocomposites, additives and applications. Appl. Sci. 2021;11:1808. doi: 10.3390/app11041808. - DOI
Publication types
Grants and funding
- 801538/CONEX-Plus program funded by Universidad Carlos III de Madrid (UC3M) and the European Commission through the Marie-Sklodowska Curie COFUND Action
- PID2020-112713RB-C22/Ministerio de Ciencia e Innovación of Spain
- 2012/00130/004/Fondos de Investigación of Fco. Javier González Benito Universidad Carlos III de Madrid
- 2011/00287/003/Universidad Carlos III de Madrid Action in Multifunctional Nanocomposite Materials
LinkOut - more resources
Full Text Sources
Miscellaneous

