Title IgA Nephropathy and Oral Bacterial Species Related to Dental Caries and Periodontitis
- PMID: 35054910
- PMCID: PMC8775524
- DOI: 10.3390/ijms23020725
Title IgA Nephropathy and Oral Bacterial Species Related to Dental Caries and Periodontitis
Abstract
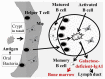
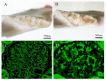
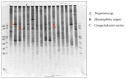
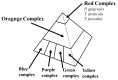
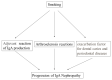
A relationship between IgA nephropathy (IgAN) and bacterial infection has been suspected. As IgAN is a chronic disease, bacteria that could cause chronic infection in oral areas might be pathogenetic bacteria candidates. Oral bacterial species related to dental caries and periodontitis should be candidates because these bacteria are well known to be pathogenic in chronic dental disease. Recently, several reports have indicated that collagen-binding protein (cnm)-(+) Streptococcs mutans is relate to the incidence of IgAN and the progression of IgAN. Among periodontal bacteria, Treponema denticola, Porphyromonas gingivalis and Campylobacte rectus were found to be related to the incidence of IgAN. These bacteria can cause IgAN-like histological findings in animal models. While the connection between oral bacterial infection, such as infection with S. mutans and periodontal bacteria, and the incidence of IgAN remains unclear, these bacterial infections might cause aberrantly glycosylated IgA1 in nasopharynx-associated lymphoid tissue, which has been reported to cause IgA deposition in mesangial areas in glomeruli, probably through the alteration of microRNAs related to the expression of glycosylation enzymes. The roles of other factors related to the incidence and progression of IgA, such as genes and cigarette smoking, can also be explained from the perspective of the relationship between these factors and oral bacteria. This review summarizes the relationship between IgAN and oral bacteria, such as cnm-(+) S. mutans and periodontal bacteria.
Keywords: IgA nephropathy; Porphyromonas gingivalis; Streptococcus mutans; cnm; dental caries; infection; mouse; oral bacteria; periodontal bacteria; periodontitis; tonsil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

