Role of Palatine Tonsil and Epipharyngeal Lymphoid Tissue in the Development of Glomerular Active Lesions (Glomerular vasculitis) in Immunoglobulin A Nephropathy
- PMID: 35054911
- PMCID: PMC8775943
- DOI: 10.3390/ijms23020727
Role of Palatine Tonsil and Epipharyngeal Lymphoid Tissue in the Development of Glomerular Active Lesions (Glomerular vasculitis) in Immunoglobulin A Nephropathy
Abstract
Hematuria is an essential symptom of immunoglobulin A nephropathy (IgAN). Although the etiology of hematuria in IgAN has not been fully elucidated, it is thought that the rupture of the glomerular basement membranes caused by intra-capillary leukocyte influx, so-called glomerular vasculitis, is the pathological condition responsible for severe hematuria. Glomerular vasculitis are active lesions that exist in the glomeruli of acute phase IgAN and it is important because it is suspected to make the transition to segmental glomerular sclerosis (SGS) as a repair scar lesion in the chronic phase, and the progression of SGS would eventually lead to glomerular obsolescence. Worsening of hematuria concomitant with acute pharyngitis is common in patients with IgAN; therefore, elucidating the relationship between the immune system of Waldeyer's ring, including the palatine tonsil and epipharyngeal lymphoid tissue, and the glomerular vasculitis may lead to understanding the nature of IgAN. The epipharynx is an immunologically activated site even under normal conditions, and enhanced activation of innate immunity is likely to occur in response to airborne infection. Hyperactivation of innate immunity via upregulation of Toll-like receptors in the interfollicular area of the palatine tonsil and epipharyngeal lymphoid tissue, followed by enhanced fractalkine/CX3CR1 interactions, appears to play an important role in the development of glomerular vasculitis in IgAN. As latent but significant epipharyngitis is present in most patients with IgAN, it is plausible that acute upper respiratory infection may contribute as a trigger for the innate epipharyngeal immune system, which is already upregulated in a chronically inflamed environment. Given that epipharyngitis and its effects on IgAN are not fully understood, we propose that the so-called "epipharynx-kidney axis" may provide an important focus for future research.
Keywords: IgA nephropathy; chronic epipharyngitis; chronic tonsillitis; epipharynx–kidney axis; glomerular vasculitis.
Conflict of interest statement
The authors do not have any financial relationships that may lead to a conflict of interest in relation to the submitted manuscript.
Figures





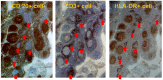



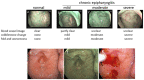

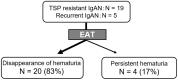


References
-
- Bellur S.S., Troyanov S., Cook H.T., Roberts I.S., Working Group of International IgA Nephropathy Network and Renal Pathology Society Immunostaining findings in IgA nephropathy: Correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol. Dial. Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

