Photic Entrainment of the Circadian System
- PMID: 35054913
- PMCID: PMC8775994
- DOI: 10.3390/ijms23020729
Photic Entrainment of the Circadian System
Abstract
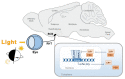
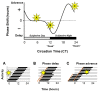
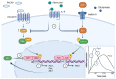
Circadian rhythms are essential for the survival of all organisms, enabling them to predict daily changes in the environment and time their behaviour appropriately. The molecular basis of such rhythms is the circadian clock, a self-sustaining molecular oscillator comprising a transcriptional-translational feedback loop. This must be continually readjusted to remain in alignment with the external world through a process termed entrainment, in which the phase of the master circadian clock in the suprachiasmatic nuclei (SCN) is adjusted in response to external time cues. In mammals, the primary time cue, or "zeitgeber", is light, which inputs directly to the SCN where it is integrated with additional non-photic zeitgebers. The molecular mechanisms underlying photic entrainment are complex, comprising a number of regulatory factors. This review will outline the photoreception pathways mediating photic entrainment, and our current understanding of the molecular pathways that drive it in the SCN.
Keywords: SCN; circadian; clock; entrainment; light; zeitgeber.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

