Aurora Kinase A Is Involved in Controlling the Localization of Aquaporin-2 in Renal Principal Cells
- PMID: 35054947
- PMCID: PMC8776063
- DOI: 10.3390/ijms23020763
Aurora Kinase A Is Involved in Controlling the Localization of Aquaporin-2 in Renal Principal Cells
Abstract
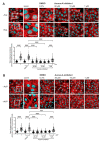
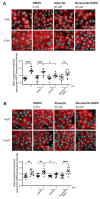
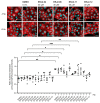
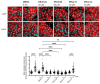
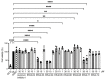
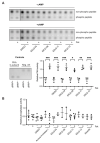
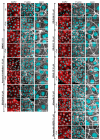
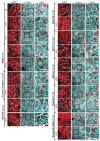
The cAMP-dependent aquaporin-2 (AQP2) redistribution from intracellular vesicles into the plasma membrane of renal collecting duct principal cells induces water reabsorption and fine-tunes body water homeostasis. However, the mechanisms controlling the localization of AQP2 are not understood in detail. Using immortalized mouse medullary collecting duct (MCD4) and primary rat inner medullary collecting duct (IMCD) cells as model systems, we here discovered a key regulatory role of Aurora kinase A (AURKA) in the control of AQP2. The AURKA-selective inhibitor Aurora-A inhibitor I and novel derivatives as well as a structurally different inhibitor, Alisertib, prevented the cAMP-induced redistribution of AQP2. Aurora-A inhibitor I led to a depolymerization of actin stress fibers, which serve as tracks for the translocation of AQP2-bearing vesicles to the plasma membrane. The phosphorylation of cofilin-1 (CFL1) inactivates the actin-depolymerizing function of CFL1. Aurora-A inhibitor I decreased the CFL1 phosphorylation, accounting for the removal of the actin stress fibers and the inhibition of the redistribution of AQP2. Surprisingly, Alisertib caused an increase in actin stress fibers and did not affect CFL1 phosphorylation, indicating that AURKA exerts its control over AQP2 through different mechanisms. An involvement of AURKA and CFL1 in the control of the localization of AQP2 was hitherto unknown.
Keywords: AQP2; AURKA; AVP; actin cytoskeleton; cofilin-1.
Conflict of interest statement
B.-T.B. is the co-founder and CEO of CELLinib GmbH (Frankfurt am Main, Germany). However, there is no conflict of interest.
Figures





References
-
- Klussmann E., Tamma G., Lorenz D., Wiesner B., Maric K., Hofmann F., Aktories K., Valenti G., Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 2001;276:20451–20457. doi: 10.1074/jbc.M010270200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

