PAR4-Mediated PI3K/Akt and RhoA/ROCK Signaling Pathways Are Essential for Thrombin-Induced Morphological Changes in MEG-01 Cells
- PMID: 35054966
- PMCID: PMC8775998
- DOI: 10.3390/ijms23020776
PAR4-Mediated PI3K/Akt and RhoA/ROCK Signaling Pathways Are Essential for Thrombin-Induced Morphological Changes in MEG-01 Cells
Abstract
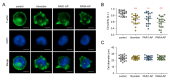
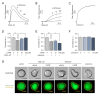
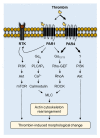
Thrombin stimulates platelets via a dual receptor system of protease-activated receptors (PARs): PAR1 and PAR4. PAR1 activation induces a rapid and transient signal associated with the initiation of platelet aggregation, whereas PAR4 activation results in a prolonged signal, required for later phases, that regulates the stable formation of thrombus. In this study, we observed differential signaling pathways for thrombin-induced PAR1 and PAR4 activation in a human megakaryoblastic leukemia cell line, MEG-01. Interestingly, thrombin induced both calcium signaling and morphological changes in MEG-01 cells via the activation of PAR1 and PAR4, and these intracellular events were very similar to those observed in platelets shown in previous studies. We developed a novel image-based assay to quantitatively measure the morphological changes in living cells, and observed the underlying mechanism for PAR1- and PAR4-mediated morphological changes in MEG-01 cells. Selective inhibition of PAR1 and PAR4 by vorapaxar and BMS-986120, respectively, showed that thrombin-induced morphological changes were primarily mediated by PAR4 activation. Treatment of a set of kinase inhibitors and 2-aminoethoxydiphenyl borate (2-APB) revealed that thrombin-mediated morphological changes were primarily regulated by calcium-independent pathways and PAR4 activation-induced PI3K/Akt and RhoA/ROCK signaling pathways in MEG-01 cells. These results indicate the importance of PAR4-mediated signaling pathways in thrombin-induced morphological changes in MEG-01 cells and provide a useful in vitro cellular model for platelet research.
Keywords: MEG-01; PAR1; PAR4; morphological change; platelet; thrombin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

