Dosing Therapeutic Radiopharmaceuticals in Obese Patients
- PMID: 35055005
- PMCID: PMC8775906
- DOI: 10.3390/ijms23020818
Dosing Therapeutic Radiopharmaceuticals in Obese Patients
Abstract
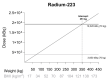
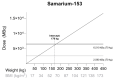
The prevalence of obesity has increased dramatically in the Western population. Obesity is known to influence not only the proportion of adipose tissue but also physiological processes that could alter drug pharmacokinetics. Yet, there are no specific dosing recommendations for radiopharmaceuticals in this patient population. This could potentially lead to underdosing and thus suboptimal treatment in obese patients, while it could also lead to drug toxicity due to high levels of radioactivity. In this review, relevant literature is summarized on radiopharmaceutical dosing and pharmacokinetic properties, and we aimed to translate these data into practical guidelines for dosing of radiopharmaceuticals in obese patients. For radium-223, dosing in obese patients is well established. Furthermore, for samarium-153-ethylenediaminetetramethylene (EDTMP), dose-escalation studies show that the maximum tolerated dose will probably not be reached in obese patients when dosing on MBq/kg. On the other hand, there is insufficient evidence to support dose recommendations in obese patients for rhenium-168-hydroxyethylidene diphosphonate (HEDP), sodium iodide-131, iodide 131-metaiodobenzylguanidine (MIBG), lutetium-177-dotatate, and lutetium-177-prostate-specific membrane antigen (PSMA). From a pharmacokinetic perspective, fixed dosing may be appropriate for these drugs. More research into obese patient populations is needed, especially in the light of increasing prevalence of obesity worldwide.
Keywords: obesity; pharmacokinetics; radiopharmaceutical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organisation Obesity and Overweight. [(accessed on 3 January 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#....
-
- Cho S.J., Yoon I.S., Kim D.D. Obesity-Related Physiological Changes and Their Pharmacokinetic Consequences. J. Pharm. Investig. 2013;43:161–169. doi: 10.1007/s40005-013-0073-4. - DOI
-
- Kratochwil C., Fendler W.P., Eiber M., Baum R., Bozkurt M.F., Czernin J., Delgado Bolton R.C., Ezziddin S., Forrer F., Hicks R.J., et al. EANM Procedure Guidelines for Radionuclide Therapy with 177Lu-Labelled PSMA-Ligands (177Lu-PSMA-RLT) Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2536–2544. doi: 10.1007/s00259-019-04485-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

