Chebulinic Acid Suppresses Adipogenesis in 3T3-L1 Preadipocytes by Inhibiting PPP1CB Activity
- PMID: 35055051
- PMCID: PMC8775935
- DOI: 10.3390/ijms23020865
Chebulinic Acid Suppresses Adipogenesis in 3T3-L1 Preadipocytes by Inhibiting PPP1CB Activity
Abstract
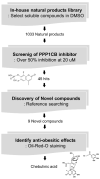
Depletion of protein phosphatase-1 catalytic subunit beta (PPP1CB), a serine/threonine protein phosphatase and potent adipogenic activator, suppresses the differentiation of 3T3-L1 preadipocytes into mature adipocytes. Therefore, PPP1CB is considered as a potential therapeutic target for obesity. We screened 1033 natural products for PPP1CB inhibitors and identified chebulinic acid, which is abundantly present in the seeds of Euphoria longana and fruits of Terminalia chebula. Chebulinic acid strongly inhibited the hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate by PPP1CB (IC50 = 300 nM) and demonstrated potent antiadipogenic effects in 3T3-L1 preadipocytes in a concentration-dependent manner. Additional studies have demonstrated that chebulinic acid suppresses early differentiation by downregulating key transcription factors that control adipogenesis in 3T3-L1 cells. These results suggested that chebulinic acid may be a potential therapeutic agent for treating obesity by inhibiting PPP1CB activity.
Keywords: 3T3-L1 adipocyte; PPP1CB; adipogenesis; chebulinic acid; natural product; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

