Therapeutic CFTR Correction Normalizes Systemic and Lung-Specific S1P Level Alterations Associated with Heart Failure
- PMID: 35055052
- PMCID: PMC8777932
- DOI: 10.3390/ijms23020866
Therapeutic CFTR Correction Normalizes Systemic and Lung-Specific S1P Level Alterations Associated with Heart Failure
Abstract
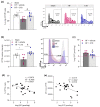
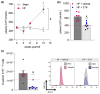
Heart failure (HF) is among the main causes of death worldwide. Alterations of sphingosine-1-phosphate (S1P) signaling have been linked to HF as well as to target organ damage that is often associated with HF. S1P's availability is controlled by the cystic fibrosis transmembrane regulator (CFTR), which acts as a critical bottleneck for intracellular S1P degradation. HF induces CFTR downregulation in cells, tissues and organs, including the lung. Whether CFTR alterations during HF also affect systemic and tissue-specific S1P concentrations has not been investigated. Here, we set out to study the relationship between S1P and CFTR expression in the HF lung. Mice with HF, induced by myocardial infarction, were treated with the CFTR corrector compound C18 starting ten weeks post-myocardial infarction for two consecutive weeks. CFTR expression, S1P concentrations, and immune cell frequencies were determined in vehicle- and C18-treated HF mice and sham controls using Western blotting, flow cytometry, mass spectrometry, and qPCR. HF led to decreased pulmonary CFTR expression, which was accompanied by elevated S1P concentrations and a pro-inflammatory state in the lungs. Systemically, HF associated with higher S1P plasma levels compared to sham-operated controls and presented with higher S1P receptor 1-positive immune cells in the spleen. CFTR correction with C18 attenuated the HF-associated alterations in pulmonary CFTR expression and, hence, led to lower pulmonary S1P levels, which was accompanied by reduced lung inflammation. Collectively, these data suggest an important role for the CFTR-S1P axis in HF-mediated systemic and pulmonary inflammation.
Keywords: cystic fibrosis transmembrane regulator; heart failure; inflammation; lung; sphingosine-1-phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

