Lignin Nanoparticles Deliver Novel Thymine Biomimetic Photo-Adducts with Antimelanoma Activity
- PMID: 35055101
- PMCID: PMC8777952
- DOI: 10.3390/ijms23020915
Lignin Nanoparticles Deliver Novel Thymine Biomimetic Photo-Adducts with Antimelanoma Activity
Abstract
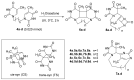
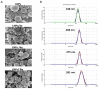
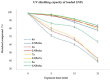
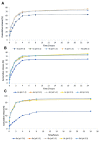
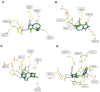
We report here the synthesis of novel thymine biomimetic photo-adducts bearing an alkane spacer between nucleobases and characterized by antimelanoma activity against two mutated cancer cell lines overexpressing human Topoisomerase 1 (TOP1), namely SKMEL28 and RPMI7951. Among them, Dewar Valence photo-adducts showed a selectivity index higher than the corresponding pyrimidine-(6-4)-pyrimidone and cyclobutane counterpart and were characterized by the highest affinity towards TOP1/DNA complex as evaluated by molecular docking analysis. The antimelanoma activity of novel photo-adducts was retained after loading into UV photo-protective lignin nanoparticles as stabilizing agent and efficient drug delivery system. Overall, these results support a combined antimelanoma and UV sunscreen strategy involving the use of photo-protective lignin nanoparticles for the controlled release of thymine dimers on the skin followed by their sacrificial transformation into photo-adducts and successive inhibition of melanoma and alert of cellular UV machinery repair pathways.
Keywords: antimelanoma activity; drug delivery; lignin nanoparticles; pyrimidine photo-adducts biomimetic approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

