Astaxanthin Exerts Anabolic Effects via Pleiotropic Modulation of the Excitable Tissue
- PMID: 35055102
- PMCID: PMC8778848
- DOI: 10.3390/ijms23020917
Astaxanthin Exerts Anabolic Effects via Pleiotropic Modulation of the Excitable Tissue
Abstract
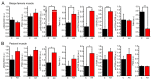
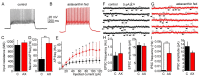
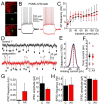
Astaxanthin is a lipid-soluble carotenoid influencing lipid metabolism, body weight, and insulin sensitivity. We provide a systematic analysis of acute and chronic effects of astaxanthin on different organs. Changes by chronic astaxanthin feeding were analyzed on general metabolism, expression of regulatory proteins in the skeletal muscle, as well as changes of excitation and synaptic activity in the hypothalamic arcuate nucleus of mice. Acute responses were also tested on canine cardiac muscle and different neuronal populations of the hypothalamic arcuate nucleus in mice. Dietary astaxanthin significantly increased food intake. It also increased protein levels affecting glucose metabolism and fatty acid biosynthesis in skeletal muscle. Inhibitory inputs innervating neurons of the arcuate nucleus regulating metabolism and food intake were strengthened by both acute and chronic astaxanthin treatment. Astaxanthin moderately shortened cardiac action potentials, depressed their plateau potential, and reduced the maximal rate of depolarization. Based on its complex actions on metabolism and food intake, our data support the previous findings that astaxanthin is suitable for supplementing the diet of patients with disturbances in energy homeostasis.
Keywords: arcuate nucleus; astaxanthin; cardiac action potential; excitability; food intake; gene expression; inhibitory postsynaptic current; metabolism; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
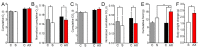


Similar articles
-
Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification.Biochem Biophys Res Commun. 2008 Feb 22;366(4):892-7. doi: 10.1016/j.bbrc.2007.12.019. Epub 2007 Dec 17. Biochem Biophys Res Commun. 2008. PMID: 18082622
-
Astaxanthin ameliorates dexamethasone-induced skeletal muscle atrophy and disorders of glucolipid metabolism.Biochem Biophys Res Commun. 2025 Jan;743:151138. doi: 10.1016/j.bbrc.2024.151138. Epub 2024 Dec 4. Biochem Biophys Res Commun. 2025. PMID: 39673970
-
An intervention study in obese mice with astaxanthin, a marine carotenoid--effects on insulin signaling and pro-inflammatory cytokines.Food Funct. 2012 Feb;3(2):120-6. doi: 10.1039/c1fo10161g. Epub 2011 Nov 17. Food Funct. 2012. PMID: 22089895
-
Impact of leucine on energy balance.J Physiol Biochem. 2013 Mar;69(1):155-63. doi: 10.1007/s13105-012-0170-2. Epub 2012 Apr 26. J Physiol Biochem. 2013. PMID: 22535285 Review.
-
Hypothalamic integration of immune function and metabolism.Prog Brain Res. 2006;153:367-405. doi: 10.1016/S0079-6123(06)53022-5. Prog Brain Res. 2006. PMID: 16876587 Free PMC article. Review.
References
-
- Nakao R., Nelson O.L., Park J.S., Mathison B.D., Thompson P.A., Chew B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010;30:2721–2725. - PubMed
-
- Baralic Andjelkovic M., Djordjevic B., Dikic N., Radivojevic N., Suzin-Zivkovic V., Radojevic-Skodric S., Pejic S. Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid. -Based Complement. Altern. Med. 2015;2015:783761. doi: 10.1155/2015/783761. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- GINOP-2.3.2-15-2016-00040/National Research, Development and Innovation Office of Hungary
- NKFI FK 134684/National Research, Development and Innovation Office of Hungary
- NKFIH PD-128370/National Research, Development and Innovation Office of Hungary
- EFOP-3.6.3-VEKOP-16-2017-00009/National Research, Development and Innovation Office of Hungary
- TKP2020-IKA-04/National Research, Development and Innovation Fund of Hungary
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

