Chemerin Affects P4 and E2 Synthesis in the Porcine Endometrium during Early Pregnancy
- PMID: 35055130
- PMCID: PMC8781843
- DOI: 10.3390/ijms23020945
Chemerin Affects P4 and E2 Synthesis in the Porcine Endometrium during Early Pregnancy
Abstract
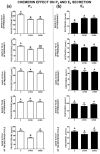
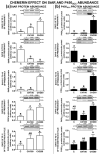
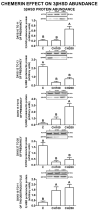
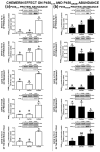
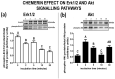
Chemerin, belonging to the adipokine family, exhibits pleiotropic activity. We hypothesised that the adipokine could be involved in the regulation of steroidogenesis in the porcine endometrium. Thus, the aim of this study was to determine the effect of chemerin on the key steroidogenic enzyme proteins' abundance (Western blot), as well as on P4 and E2 secretion (radioimmunoassay) by the porcine endometrium during early pregnancy and the mid-luteal phase of the oestrous cycle. Moreover, we investigated the hormone impact on Erk and Akt signalling pathway activation (Western blot). Chemerin stimulated E2 production on days 10 to 11 of pregnancy. On days 10 to 11 and 15 to 16 of gestation, and on days 10 to 11 of the cycle, chemerin enhanced the expression of StAR and all steroidogenic enzyme proteins. On days 12 to 13 of pregnancy, chemerin decreased StAR and most of the steroidogenic enzyme proteins' abundance, whereas the P450C17 abundance was increased. On days 27 to 28 of pregnancy, chemerin increased StAR and P450C17 protein contents and decreased 3βHSD protein amounts. It was noted that the adipokine inhibited Erk1/2 and stimulated Akt phosphorylation. The obtained results indicate that chemerin affected P4 and E2 synthesis through the Erk1/2 and Akt signalling pathways.
Keywords: chemerin; endometrium; gestation; pig; signalling pathways; steroidogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The effect of oestradiol and progesterone on chemerin system expression in the porcine endometrium during early pregnancy and the mid-luteal phase of the oestrous cycle.Theriogenology. 2023 Jan 15;196:186-201. doi: 10.1016/j.theriogenology.2022.11.011. Epub 2022 Nov 12. Theriogenology. 2023. PMID: 36423513
-
Chemerin as a modulator of ovarian steroidogenesis in pigs: an in vitro study.Theriogenology. 2021 Jan 15;160:95-101. doi: 10.1016/j.theriogenology.2020.10.040. Epub 2020 Nov 5. Theriogenology. 2021. PMID: 33189079
-
Role of resistin in the porcine uterus: effects on endometrial steroidogenesis.Reprod Fertil Dev. 2025 Jan;37:RD24097. doi: 10.1071/RD24097. Reprod Fertil Dev. 2025. PMID: 39786988
-
Adiponectin affects uterine steroidogenesis during early pregnancy and the oestrous cycle: An in vitro study.Anim Reprod Sci. 2022 Oct;245:107067. doi: 10.1016/j.anireprosci.2022.107067. Epub 2022 Sep 6. Anim Reprod Sci. 2022. PMID: 36113273
-
Aspects of placental estrogen synthesis in the pig.Exp Clin Endocrinol. 1994;102(3):175-84. doi: 10.1055/s-0029-1211279. Exp Clin Endocrinol. 1994. PMID: 7995337 Review.
Cited by
-
Chemerin's Role in Endometrial Dysfunction: Insights From Transcriptomic Analysis.J Cell Mol Med. 2025 Mar;29(6):e70417. doi: 10.1111/jcmm.70417. J Cell Mol Med. 2025. PMID: 40117172 Free PMC article.
-
Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis.Cells. 2022 Mar 30;11(7):1161. doi: 10.3390/cells11071161. Cells. 2022. PMID: 35406725 Free PMC article.
-
Effect of Vitamin D3 on Chemerin and Adiponectin Levels in Uterus of Polycystic Ovary Syndrome Rats.Cells. 2023 Aug 8;12(16):2026. doi: 10.3390/cells12162026. Cells. 2023. PMID: 37626836 Free PMC article.
References
-
- Smolinska N., Kiezun M., Dobrzyn K., Rytelewska E., Kisielewska K., Gudelska M., Zaobidna E., Bogus-Nowakowska K., Wyrebek J., Bors K., et al. Expression of Chemerin and Its Receptors in the Porcine Hypothalamus and Plasma Chemerin Levels during the Oestrous Cycle and Early Pregnancy. Int J. Mol. Sci. 2019;20:3887. doi: 10.3390/ijms20163887. - DOI - PMC - PubMed
-
- Kisielewska K., Rytelewska E., Gudelska M., Kiezun M., Dobrzyn K., Bogus-Nowakowska K., Kaminska B., Smolinska N., Kaminski T. Relative abundance of chemerin mRNA transcript and protein in pituitaries of pigs during the estrous cycle and early pregnancy and associations with LH and FSH secretion during the estrous cycle. Anim. Reprod Sci. 2020;219:106532. doi: 10.1016/j.anireprosci.2020.106532. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

