Molecular Mechanisms Leading from Periodontal Disease to Cancer
- PMID: 35055157
- PMCID: PMC8778447
- DOI: 10.3390/ijms23020970
Molecular Mechanisms Leading from Periodontal Disease to Cancer
Abstract
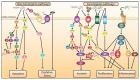
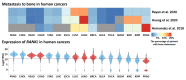
Periodontitis is prevalent in half of the adult population and raises critical health concerns as it has been recently associated with an increased risk of cancer. While information about the topic remains somewhat scarce, a deeper understanding of the underlying mechanistic pathways promoting neoplasia in periodontitis patients is of fundamental importance. This manuscript presents the literature as well as a panel of tables and figures on the molecular mechanisms of Porphyromonas gingivalis and Fusobacterium nucleatum, two main oral pathogens in periodontitis pathology, involved in instigating tumorigenesis. We also present evidence for potential links between the RANKL-RANK signaling axis as well as circulating cytokines/leukocytes and carcinogenesis. Due to the nonconclusive data associating periodontitis and cancer reported in the case and cohort studies, we examine clinical trials relevant to the topic and summarize their outcome.
Keywords: Fusobacterium nucleatum; Porphyromonas gingivalis; RANK ligand; cancer; immune response; periodontal disease; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Regional Office for Europe [(accessed on 27 November 2021)]. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/oral-health....
-
- Mehrotra N., Singh S. Periodontitis. StatPearls Publishing; Treasure Island, FL, USA: 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

