Regulation of the Receptor Tyrosine Kinase AXL in Response to Therapy and Its Role in Therapy Resistance in Glioblastoma
- PMID: 35055167
- PMCID: PMC8781963
- DOI: 10.3390/ijms23020982
Regulation of the Receptor Tyrosine Kinase AXL in Response to Therapy and Its Role in Therapy Resistance in Glioblastoma
Abstract
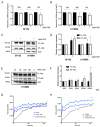
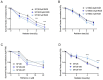
The receptor tyrosine kinase AXL (RTK-AXL) is implicated in therapy resistance and tumor progression in glioblastoma multiforme (GBM). Here, we investigated therapy-induced receptor modifications and how endogenous RTK-AXL expression and RTK-AXL inhibition contribute to therapy resistance in GBM. GBM cell lines U118MG and SF126 were exposed to temozolomide (TMZ) and radiation (RTX). Receptor modifications in response to therapy were investigated on protein and mRNA levels. TMZ-resistant and RTK-AXL overexpressing cell lines were exposed to increasing doses of TMZ and RTX, with and without RTK-AXL tyrosine kinase inhibitor (TKI). Colorimetric microtiter (MTT) assay and colony formation assay (CFA) were used to assess cell viability. Results showed that the RTK-AXL shedding product, C-terminal AXL (CT-AXL), rises in response to repeated TMZ doses and under hypoxia, acts as a surrogate marker for radio-resistance. Endogenous RTX-AXL overexpression leads to therapy resistance, whereas combination therapy of TZM and RTX with TKI R428 significantly increases therapeutic effects. This data proves the role of RTK-AXL in acquired and intrinsic therapy resistance. By demonstrating that therapy resistance may be overcome by combining AXL TKI with standard treatments, we have provided a rationale for future study designs investigating AXL TKIs in GBM.
Keywords: R428; RTK-AXL; glioblastoma multiforme; post-translational receptor modification; radiation; temozolomide; tyrosine kinase inhibitor (TKI).
Conflict of interest statement
No conflicts of interest exist in the submission of the manuscript and the manuscript is approved by all authors for publication.
Figures


References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
-
- Grisanti S., Ferrari V.D., Buglione M., Agazzi G.M., Liserre R., Poliani L., Buttolo L., Gipponi S., Pedersini R., Consoli F., et al. Second line treatment of recurrent glioblastoma with sunitinib: Results of a phase II study and systematic review of literature. J. Neurosurg. Sci. 2019;63:458–467. doi: 10.23736/S0390-5616.16.03874-1. - DOI - PubMed
-
- Lombardi G., De Salvo G.L., Brandes A.A., Eoli M., Rudà R., Faedi M., Lolli I., Pace A., Daniele B., Pasqualetti F., et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20:110–119. doi: 10.1016/S1470-2045(18)30675-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

