Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy
- PMID: 35055222
- PMCID: PMC8780348
- DOI: 10.3390/nano12020205
Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy
Abstract
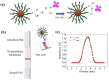
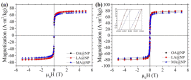
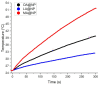
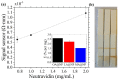
Today, public health is one of the most important challenges in society. Cancer is the leading cause of death, so early diagnosis and localized treatments that minimize side effects are a priority. Magnetic nanoparticles have shown great potential as magnetic resonance imaging contrast agents, detection tags for in vitro biosensing, and mediators of heating in magnetic hyperthermia. One of the critical characteristics of nanoparticles to adjust to the biomedical needs of each application is their polymeric coating. Fatty acid coatings are known to contribute to colloidal stability and good surface crystalline quality. While monolayer coatings make the particles hydrophobic, a fatty acid double-layer renders them hydrophilic, and therefore suitable for use in body fluids. In addition, they provide the particles with functional chemical groups that allow their bioconjugation. This work analyzes three types of self-assembled bilayer fatty acid coatings of superparamagnetic iron oxide nanoparticles: oleic, lauric, and myristic acids. We characterize the particles magnetically and structurally and study their potential for resonance imaging, magnetic hyperthermia, and labeling for biosensing in lateral flow immunoassays. We found that the myristic acid sample reported a large r2 relaxivity, superior to existing iron-based commercial agents. For magnetic hyperthermia, a significant specific absorption rate value was obtained for the oleic sample. Finally, the lauric acid sample showed promising results for nanolabeling.
Keywords: biosensor; inductive sensing; lateral flow immunoassays; magnetic hyperthermia; magnetic nanoparticles; magnetic relaxation; magnetic resonance imaging.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Blay J.Y., Boucher S., Le Vu B., Cropet C., Chabaud S., Perol D., Barranger E., Campone M., Conroy T., Coutant C., et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6:100134. doi: 10.1016/j.esmoop.2021.100134. - DOI - PMC - PubMed
-
- Chun P. Lateral Flow Immunoassay. Humana Press; New York, NY, USA: 2009. Colloidal Gold and Other Labels for Lateral Flow Immunoassays; pp. 1–19.
Grants and funding
- MAT2017-84959-C2-1-R/Spanish Ministry of Economy and Competitiveness
- IDI/2018/000185/Gobierno del Principado de Asturias
- Ferro-Tera/Romanian Academy and Italian National research Council
- JNIR-RO grant 04-4-1142-2021/2025 - item 34 - JINR Order no. 365/11.05.2021/Romanian Ministry of Research
- RA-TB/CFATR/LMF/Romanian Ministry of Research
LinkOut - more resources
Full Text Sources

