Nanotechnologies in Delivery of DNA and mRNA Vaccines to the Nasal and Pulmonary Mucosa
- PMID: 35055244
- PMCID: PMC8777913
- DOI: 10.3390/nano12020226
Nanotechnologies in Delivery of DNA and mRNA Vaccines to the Nasal and Pulmonary Mucosa
Abstract
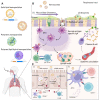
Recent advancements in the field of in vitro transcribed mRNA (IVT-mRNA) vaccination have attracted considerable attention to such vaccination as a cutting-edge technique against infectious diseases including COVID-19 caused by SARS-CoV-2. While numerous pathogens infect the host through the respiratory mucosa, conventional parenterally administered vaccines are unable to induce protective immunity at mucosal surfaces. Mucosal immunization enables the induction of both mucosal and systemic immunity, efficiently removing pathogens from the mucosa before an infection occurs. Although respiratory mucosal vaccination is highly appealing, successful nasal or pulmonary delivery of nucleic acid-based vaccines is challenging because of several physical and biological barriers at the airway mucosal site, such as a variety of protective enzymes and mucociliary clearance, which remove exogenously inhaled substances. Hence, advanced nanotechnologies enabling delivery of DNA and IVT-mRNA to the nasal and pulmonary mucosa are urgently needed. Ideal nanocarriers for nucleic acid vaccines should be able to efficiently load and protect genetic payloads, overcome physical and biological barriers at the airway mucosal site, facilitate transfection in targeted epithelial or antigen-presenting cells, and incorporate adjuvants. In this review, we discuss recent developments in nucleic acid delivery systems that target airway mucosa for vaccination purposes.
Keywords: DNA vaccine; intranasal delivery; mRNA vaccine; mucosal immune response; nanoparticles; pulmonary delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hajj K.A., Whitehead K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:17056. doi: 10.1038/natrevmats.2017.56. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

