Au Nanoparticle-Based Amplified DNA Detection on Poly-l-lysine Monolayer-Functionalized Electrodes
- PMID: 35055260
- PMCID: PMC8780787
- DOI: 10.3390/nano12020242
Au Nanoparticle-Based Amplified DNA Detection on Poly-l-lysine Monolayer-Functionalized Electrodes
Abstract
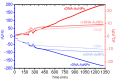
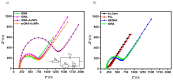
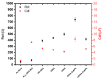
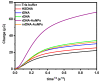
Affinity sensing of nucleic acids is among the most investigated areas in biosensing due to the growing importance of DNA diagnostics in healthcare research and clinical applications. Here, we report a simple electrochemical DNA detection layer, based on poly-l-lysine (PLL), in combination with gold nanoparticles (AuNPs) as a signal amplifier. The layer shows excellent reduction of non-specific binding and thereby high contrast between amplified and non-amplified signals with functionalized AuNPs; the relative change in current was 10-fold compared to the non-amplified signal. The present work may provide a general method for the detection of tumor markers based on electrochemical DNA sensing.
Keywords: AuNPs; DNA sensing; electrochemical biosensor; poly-l-lysine; signal amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dual-amplified strategy for ultrasensitive electrochemical biosensor based on click chemistry-mediated enzyme-assisted target recycling and functionalized fullerene nanoparticles in the detection of microRNA-141.Biosens Bioelectron. 2020 Feb 15;150:111964. doi: 10.1016/j.bios.2019.111964. Epub 2019 Dec 13. Biosens Bioelectron. 2020. PMID: 31929092
-
Amplified electrochemical antibiotic aptasensing based on electrochemically deposited AuNPs coordinated with PEI-functionalized Fe-based metal-organic framework.Mikrochim Acta. 2021 Aug 4;188(8):286. doi: 10.1007/s00604-021-04912-z. Mikrochim Acta. 2021. PMID: 34345968
-
An ultrasensitive signal-on electrochemical aptasensor for ochratoxin A determination based on DNA controlled layer-by-layer assembly of dual gold nanoparticle conjugates.Biosens Bioelectron. 2018 Oct 15;117:845-851. doi: 10.1016/j.bios.2018.07.012. Epub 2018 Jul 11. Biosens Bioelectron. 2018. PMID: 30096739
-
Ru(bpy)32+-Silica@Poly-L-lysine-Au as labels for electrochemiluminescence lysozyme aptasensor based on 3D graphene.Biosens Bioelectron. 2018 May 30;106:50-56. doi: 10.1016/j.bios.2018.01.059. Epub 2018 Jan 31. Biosens Bioelectron. 2018. PMID: 29414088
-
Gold nanoparticle-based signal amplification for biosensing.Anal Biochem. 2011 Oct 1;417(1):1-16. doi: 10.1016/j.ab.2011.05.027. Epub 2011 May 26. Anal Biochem. 2011. PMID: 21703222 Review.
Cited by
-
The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review.Molecules. 2025 Jun 23;30(13):2708. doi: 10.3390/molecules30132708. Molecules. 2025. PMID: 40649227 Free PMC article. Review.
References
-
- Srisombat L., Jamison A.C., Lee T.R. Stability: A key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants. Coll. Surf. A Physicochem. Eng. Asp. 2011;390:1–19. doi: 10.1016/j.colsurfa.2011.09.020. - DOI
-
- Shivashankar M., Vinodini V.R., Mishra P., Uma K. Applications of implantable medical sensors for heart failure: A review. Int. J. Pharm. Pharm. Sci. 2014;6:1–5.
Grants and funding
LinkOut - more resources
Full Text Sources

