Contemporary Molecular Analyses of Malignant Tumors for Precision Treatment and the Implication in Oral Squamous Cell Carcinoma
- PMID: 35055327
- PMCID: PMC8780757
- DOI: 10.3390/jpm12010012
Contemporary Molecular Analyses of Malignant Tumors for Precision Treatment and the Implication in Oral Squamous Cell Carcinoma
Abstract
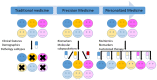
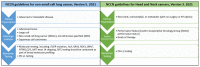
New molecular tests and methods, in addition to morphology-based diagnosis, are widely used as a new standard of care in many tumors. "One-size-fits-all medicine" is now shifting to precision medicine. This review is intended to discuss the key steps toward to development of precision medicine and its implication in oral squamous cell carcinoma. The challenges and opportunities of precision medicine in oral cancer will be sequentially discussed based on the four steps of precision medicine: identification/detection, diagnosis, treatment and monitoring.
Keywords: oral squamous cell carcinoma; personalized medicine; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Biomarkers: paving stones on the road towards the personalized precision medicine for oral squamous cell carcinoma.BMC Cancer. 2018 Sep 21;18(1):911. doi: 10.1186/s12885-018-4806-7. BMC Cancer. 2018. PMID: 30241505 Free PMC article. Review.
-
Toward the use of precision medicine for the treatment of head and neck squamous cell carcinoma.Oncotarget. 2017 Jan 10;8(2):2141-2152. doi: 10.18632/oncotarget.13798. Oncotarget. 2017. PMID: 27924064 Free PMC article. Review.
-
Application of Multimodality Imaging Fusion Technology in Diagnosis and Treatment of Malignant Tumors under the Precision Medicine Plan.Chin Med J (Engl). 2016 Dec 20;129(24):2991-2997. doi: 10.4103/0366-6999.195467. Chin Med J (Engl). 2016. PMID: 27958232 Free PMC article. Review.
-
Oral Cancer: Genetics and the Role of Precision Medicine.Surg Oncol Clin N Am. 2020 Jan;29(1):127-144. doi: 10.1016/j.soc.2019.08.010. Surg Oncol Clin N Am. 2020. PMID: 31757309 Review.
-
Oral Cancer: Genetics and the Role of Precision Medicine.Dent Clin North Am. 2018 Jan;62(1):29-46. doi: 10.1016/j.cden.2017.08.002. Dent Clin North Am. 2018. PMID: 29126492 Review.
Cited by
-
The altered composition of gut microbiota and biochemical features as well as dietary patterns in a southern Chinese population with recurrent renal calcium oxalate stones.Urolithiasis. 2023 Jul 17;51(1):95. doi: 10.1007/s00240-023-01467-x. Urolithiasis. 2023. PMID: 37458823
-
Associations between lifestyle factors, physiological conditions, and epigenetic age acceleration in an Asian population.Biogerontology. 2025 Feb 5;26(2):51. doi: 10.1007/s10522-025-10195-1. Biogerontology. 2025. PMID: 39907822 Free PMC article.
-
Integrated Proteomics Based on 2D Gel Electrophoresis and Mass Spectrometry with Validations: Identification of a Biomarker Compendium for Oral Submucous Fibrosis-An Indian Study.J Pers Med. 2022 Feb 3;12(2):208. doi: 10.3390/jpm12020208. J Pers Med. 2022. PMID: 35207696 Free PMC article.
-
Editorial of Special Issue "Oral Cancer: From Pathophysiology to Novel Therapeutic Approaches".Biomedicines. 2023 Oct 11;11(10):2748. doi: 10.3390/biomedicines11102748. Biomedicines. 2023. PMID: 37893121 Free PMC article.
-
Evolving trends in oral cancer burden in Europe: a systematic review.Front Oncol. 2024 Oct 18;14:1444326. doi: 10.3389/fonc.2024.1444326. eCollection 2024. Front Oncol. 2024. PMID: 39493458 Free PMC article.
References
-
- Margalit D.N., Sacco A.G., Cooper J.S., Ridge J.A., Bakst R.L., Beadle B.M., Beitler J.J., Chang S.S., Chen A.M., Galloway T.J., et al. Systematic review of postoperative therapy for resected squamous cell carcinoma of the head and neck: Executive summary of the American Radium Society appropriate use criteria. Head Neck. 2021;43:367–391. doi: 10.1002/hed.26490. - DOI - PMC - PubMed
-
- Sacco A.G., Chen R., Worden F.P., Wong D.J.L., Adkins D., Swiecicki P., Chai-Ho W., Oppelt P., Ghosh D., Bykowski J., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

