Clinical, Genetic and Functional Characterization of a Novel AVPR2 Missense Mutation in a Woman with X-Linked Recessive Nephrogenic Diabetes Insipidus
- PMID: 35055433
- PMCID: PMC8779739
- DOI: 10.3390/jpm12010118
Clinical, Genetic and Functional Characterization of a Novel AVPR2 Missense Mutation in a Woman with X-Linked Recessive Nephrogenic Diabetes Insipidus
Abstract
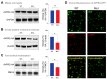
Nephrogenic diabetes insipidus (NDI) is a rare disorder characterized by renal unresponsiveness to the hormone vasopressin, leading to excretion of large volumes of diluted urine. Mutations in the arginine vasopressin receptor-2 (AVPR2) gene cause congenital NDI and have an X-linked recessive inheritance. The disorder affects almost exclusively male family members, but female carriers occasionally present partial phenotypes due to skewed inactivation of the X-chromosome. Here, we report a rare case of a woman affected with X-linked recessive NDI, presenting an average urinary output of 12 L/day. Clinical and biochemical studies showed incomplete responses to water deprivation and vasopressin stimulation tests. Genetic analyses revealed a novel heterozygous missense mutation (c.493G > C, p.Ala165Pro) in the AVPR2 gene. Using a combination of in-silico protein modeling with human cellular models and molecular phenotyping, we provide functional evidence for phenotypic effects. The mutation destabilizes the helical structure of the AVPR2 transmembrane domains and disrupts its plasma membrane localization and downstream intracellular signaling pathways upon activation with its agonist vasopressin. These defects lead to deficient aquaporin 2 (AQP2) membrane translocation, explaining the inability to concentrate urine in this patient.
Keywords: AVPR2; case report; genetics; nephrogenic diabetes insipidus; vasopressin.
Conflict of interest statement
All authors declare that they have no competing interests. The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Figures



Similar articles
-
Genetic forms of nephrogenic diabetes insipidus (NDI): Vasopressin receptor defect (X-linked) and aquaporin defect (autosomal recessive and dominant).Best Pract Res Clin Endocrinol Metab. 2016 Mar;30(2):263-76. doi: 10.1016/j.beem.2016.02.010. Epub 2016 Mar 2. Best Pract Res Clin Endocrinol Metab. 2016. PMID: 27156763 Review.
-
A Novel Missense Mutation of Arginine Vasopressin Receptor 2 in a Chinese Family with Congenital Nephrogenic Diabetes Insipidus: X-Chromosome Inactivation in Female CNDI Patients with Heterozygote 814A>G Mutation.Biomed Res Int. 2022 Jul 12;2022:7073158. doi: 10.1155/2022/7073158. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2024 Mar 20;2024:9753837. doi: 10.1155/2024/9753837. PMID: 35865667 Free PMC article. Retracted.
-
A female with X-linked Nephrogenic diabetes insipidus in a family with inherited central diabetes Insipidus: Case report and review of the literature.Am J Med Genet A. 2020 May;182(5):1032-1040. doi: 10.1002/ajmg.a.61516. Epub 2020 Feb 19. Am J Med Genet A. 2020. PMID: 32073219
-
A novel AVPR2 gene mutation in a Chinese pedigree with nephrogenic diabetes insipidus.Postgrad Med. 2024 Aug;136(6):683-690. doi: 10.1080/00325481.2024.2383555. Epub 2024 Jul 23. Postgrad Med. 2024. PMID: 39041787
-
V2R mutations and nephrogenic diabetes insipidus.Prog Mol Biol Transl Sci. 2009;89:15-29. doi: 10.1016/S1877-1173(09)89002-9. Epub 2009 Oct 7. Prog Mol Biol Transl Sci. 2009. PMID: 20374732 Review.
References
-
- Rondon-Berrios H., Berl T. Physiology and Pathophysiology of Water Homeostasis. Front. Horm. Res. 2019;52:8–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

