The Oesophageal Squamous Cell Carcinoma Cell Line COLO-680N Fails to Support Sustained Cryptosporidium parvum Proliferation
- PMID: 35055997
- PMCID: PMC8778297
- DOI: 10.3390/pathogens11010049
The Oesophageal Squamous Cell Carcinoma Cell Line COLO-680N Fails to Support Sustained Cryptosporidium parvum Proliferation
Abstract
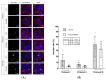
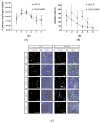
Cryptosporidium parvum is an important diarrhoea-associated protozoan, which is difficult to propagate in vitro. In 2017, a report described a continuous culture of C. parvum Moredun strain, in the oesophageal squamous cell carcinoma cell line COLO-680N, as an easy-to-use system for C. parvum propagation and continuous production of oocysts. Here, we report that-using the Köllitsch strain of C. parvum-even though COLO-680N cells, indeed, allowed parasite invasion and early asexual parasite replication, C. parvum proliferation decreased after the second day post infection. Considering recurring studies, reporting on successful production of newly generated Cryptosporidium oocysts in the past, and the subsequent replication failure by other research groups, the current data stand as a reminder of the importance of reproducibility of in vitro systems in cryptosporidiosis research. This is of special importance since it will only be possible to develop promising strategies to fight cryptosporidiosis and its ominous consequences for both human and animal health by a continuous and reliable methodological progress.
Keywords: COLO-680N; Cryptosporidium parvum; cell culture; immunofluorescence; qPCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A novel, stain-free, natural auto-fluorescent signal, Sig M, identified from cytometric and transcriptomic analysis of infectivity of Cryptosporidium hominis and Cryptosporidium parvum.Front Cell Infect Microbiol. 2023 May 22;13:1178576. doi: 10.3389/fcimb.2023.1178576. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37284498 Free PMC article.
-
A Cell Culture Platform for the Cultivation of Cryptosporidium parvum.Curr Protoc Microbiol. 2019 Jun;53(1):e80. doi: 10.1002/cpmc.80. Epub 2019 Feb 8. Curr Protoc Microbiol. 2019. PMID: 30735306
-
A cell culture platform for Cryptosporidium that enables long-term cultivation and new tools for the systematic investigation of its biology.Int J Parasitol. 2018 Mar;48(3-4):197-201. doi: 10.1016/j.ijpara.2017.10.001. Epub 2017 Nov 28. Int J Parasitol. 2018. PMID: 29195082 Free PMC article.
-
Detection of Cryptosporidium parvum in human feces by PCR.Tokai J Exp Clin Med. 1998 Dec;23(6):309-11. Tokai J Exp Clin Med. 1998. PMID: 10622627 Review.
-
Rapid review: Recent advances in in vitro models for the study of Cryptosporidium parvum.Curr Res Parasitol Vector Borne Dis. 2025 May 15;7:100269. doi: 10.1016/j.crpvbd.2025.100269. eCollection 2025. Curr Res Parasitol Vector Borne Dis. 2025. PMID: 40495915 Free PMC article. Review.
Cited by
-
A Pumpless and Tubeless Microfluidic Device Enables Extended In Vitro Development of Cryptosporidium parvum.Open Forum Infect Dis. 2024 Oct 16;11(11):ofae625. doi: 10.1093/ofid/ofae625. eCollection 2024 Nov. Open Forum Infect Dis. 2024. PMID: 39512424 Free PMC article.
-
A novel, stain-free, natural auto-fluorescent signal, Sig M, identified from cytometric and transcriptomic analysis of infectivity of Cryptosporidium hominis and Cryptosporidium parvum.Front Cell Infect Microbiol. 2023 May 22;13:1178576. doi: 10.3389/fcimb.2023.1178576. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37284498 Free PMC article.
-
Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon.Pathogens. 2025 Mar 14;14(3):287. doi: 10.3390/pathogens14030287. Pathogens. 2025. PMID: 40137772 Free PMC article.
References
-
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
-
- Amadi B., Mwiya M., Sianongo S., Payne L., Watuka A., Katubulushi M., Kelly P. High Dose Prolonged Treatment with Nitazoxanide Is Not Effective for Cryptosporidiosis in HIV Positive Zambian Children: A Randomised Controlled Trial. BMC Infect. Dis. 2009;9:195. doi: 10.1186/1471-2334-9-195. - DOI - PMC - PubMed
-
- Checkley W., White A.C., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.-M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., et al. A Review of the Global Burden, Novel Diagnostics, Therapeutics, and Vaccine Targets for Cryptosporidium. Lancet Infect. Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

