The Conserved Macrodomain Is a Potential Therapeutic Target for Coronaviruses and Alphaviruses
- PMID: 35056042
- PMCID: PMC8780475
- DOI: 10.3390/pathogens11010094
The Conserved Macrodomain Is a Potential Therapeutic Target for Coronaviruses and Alphaviruses
Abstract
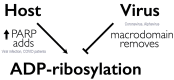
Emerging and re-emerging viral diseases pose continuous public health threats, and effective control requires a combination of non-pharmacologic interventions, treatment with antivirals, and prevention with vaccines. The COVID-19 pandemic has demonstrated that the world was least prepared to provide effective treatments. This lack of preparedness has been due, in large part, to a lack of investment in developing a diverse portfolio of antiviral agents, particularly those ready to combat viruses of pandemic potential. Here, we focus on a drug target called macrodomain that is critical for the replication and pathogenesis of alphaviruses and coronaviruses. Some mutations in alphavirus and coronaviral macrodomains are not tolerated for virus replication. In addition, the coronavirus macrodomain suppresses host interferon responses. Therefore, macrodomain inhibitors have the potential to block virus replication and restore the host's protective interferon response. Viral macrodomains offer an attractive antiviral target for developing direct acting antivirals because they are highly conserved and have a structurally well-defined (druggable) binding pocket. Given that this target is distinct from the existing RNA polymerase and protease targets, a macrodomain inhibitor may complement current approaches, pre-empt the threat of resistance and offer opportunities to develop combination therapies for combating COVID-19 and future viral threats.
Keywords: ADP-ribosylation; ADP-ribosylhydrolase; SARS-CoV-2; alphavirus; coronavirus; macrodomain; therapeutics.
Conflict of interest statement
D.E.G. is on advisory boards for Takeda Pharmaceuticals, GlaxoSmithKline, and GreenLight Biosciences. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Hoarau J.-J., Bandjee M.-C.J., Trotot P.K., Das T., Li-Pat-Yuen G., Dassa B., Denizot M., Guichard E., Ribera A., Henni T., et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

