Jakinibs of All Trades: Inhibiting Cytokine Signaling in Immune-Mediated Pathologies
- PMID: 35056105
- PMCID: PMC8779366
- DOI: 10.3390/ph15010048
Jakinibs of All Trades: Inhibiting Cytokine Signaling in Immune-Mediated Pathologies
Abstract
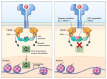
Over the last 25 years, inhibition of Janus kinases (JAKs) has been pursued as a modality for treating various immune and inflammatory disorders. While the clinical development of JAK inhibitors (jakinibs) began with the investigation of their use in allogeneic transplantation, their widest successful application came in autoimmune and allergic diseases. Multiple molecules have now been approved for diseases ranging from rheumatoid and juvenile arthritis to ulcerative colitis, atopic dermatitis, graft-versus-host-disease (GVHD) and other inflammatory pathologies in 80 countries around the world. Moreover, two jakinibs have also shown surprising efficacy in the treatment of hospitalized coronavirus disease-19 (COVID-19) patients, indicating additional roles for jakinibs in infectious diseases, cytokine storms and other hyperinflammatory syndromes. Jakinibs, as a class of pharmaceutics, continue to expand in clinical applications and with the development of more selective JAK-targeting and organ-selective delivery. Importantly, jakinib safety and pharmacokinetics have been investigated alongside clinical development, further cementing the potential benefits and limits of jakinib use. This review covers jakinibs that are approved or are under late phase investigation, focusing on clinical applications, pharmacokinetic and safety profiles, and future opportunities and challenges.
Keywords: COVID-19; JAK; autoimmune diseases; cytokines; inflammation.
Conflict of interest statement
John J. O’Shea, M.D., and the NIH hold patents related to therapeutic JAK targeting and previously had a Collaborative Research Agreement and Development Award with Pfizer. The other authors declare no financial or commercial conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources