Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity
- PMID: 35056129
- PMCID: PMC8777672
- DOI: 10.3390/ph15010072
Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity
Abstract
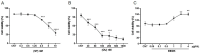
The Na+/K+-ATPase, present in the basolateral membrane of human corneal endothelial cells (HCECs), is known to play an important role for corneal transparency. Na+/K+-ATPase dysfunction is one of the major causes of corneal decompensation. The ethanol extract of Diospyros kaki (EEDK) has been reported to increase corneal cell viability. Thus, we treated HCECs with EEDK and studied its effects on HCECs survival and Na+/K+-ATPase against cytotoxic drugs like staurosporine (ST) and ouabain (OU). Firstly, survival assays, (MTT assay and live dead-imaging) showed that decreased HCECs viability by ST and OU was significantly recovered by EEDK co-treatment. Secondly, Na+/K+-ATPase activity assays revealed that EEDK enhanced Na+/K+-ATPase enzymatic activity (* p < 0.01) with/without ST and OU. Finally, Na+/K+-ATPase expression analysis (Western Blot and confocal microscopy) demonstrated that EEDK treatment with/without ST and OU facilitates Na+/K+-ATPase expression in HCECs. Taken together, our findings led us to the conclusion that EEDK might aid HCECs survival in vitro by increasing the activity and expression of Na+/K+-ATPase enzyme. Since Na+/K+-ATPase activity is important to maintain cellular function of HCECs, we suggest that EEDK can be a potential effective agent against corneal edema and related corneal disorders.
Keywords: Na+/K+-ATPase; cell viability; cornea; enzymatic activity; ethanol extract of Diospyros kaki; human corneal endothelial cells; ouabain; persimmon leaves; staurosporine.
Conflict of interest statement
The authors declare that they have no conflict of interest related to this work.
Figures





References
-
- Barry P.A., Petroll W.M., Andrews P.M., Cavanagh H.D., Jester J.V. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Investig. Ophthalmol. Vis. Sci. 1995;36:1115–1124. - PubMed
LinkOut - more resources
Full Text Sources

