Preparation of Fe3O4@PDA@Au@GO Composite as SERS Substrate and Its Application in the Enrichment and Detection for Phenanthrene
- PMID: 35056293
- PMCID: PMC8778011
- DOI: 10.3390/mi13010128
Preparation of Fe3O4@PDA@Au@GO Composite as SERS Substrate and Its Application in the Enrichment and Detection for Phenanthrene
Abstract
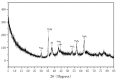
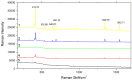
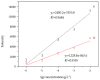
In this study, highly active Fe3O4@PDA@Au@GO surface-enhanced Raman spectroscopy (SERS) active substrate was synthesized for application in the enrichment and detection of trace polycyclic aromatic hydrocarbons (PAHs) in the environment. The morphology and structure were characterized by transmission electron microscopy (TEM), energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD) and UV-visible absorption spectrum (UV-vis spectra). The effect of each component of Fe3O4@PDA@Au@GO nanocomposites on SERS was explored, and it was found that gold nanoparticles (Au NPs) are crucial to enhance the Raman signal based on the electromagnetic enhancement mechanism, and apart from enriching the PAHs through π-π interaction, graphene oxide (GO) also generates strong chemical enhancement of Raman signals, and polydopamine (PDA) can prevent Au from shedding and agglomeration. The existence of Fe3O4 aided the quick separation of substrate from the solutions, which greatly simplified the detection procedure and facilitated the reuse of the substrate. The SERS active substrate was used to detect phenanthrene in aqueous solution with a detection limit of 10-7 g/L (5.6 × 10-10 mol/L), which is much lower than that of ordinary Raman, it is promising for application in the enrichment and detection of trace PAHs.
Keywords: PAHs; enrichment detection; nanocomposites; surface-enhanced Raman spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Chen H., Xia D., Yuan Y.X. Surface Enhanced Raman Spectroscopic Investigation of PAHs at a PDMS-Au Composite Substrate PDMS-Au. Chem. Res. 2017;38:376–382.
-
- White A.J., Bradshaw P.T., Herring A.H., Teitelbaum S.L., Beyea J., Stellman S.D., Steck S.E., Mordukhovich I., Eng S.M., Engel L.S., et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ. Int. 2016;89–90:185–192. doi: 10.1016/j.envint.2016.02.009. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

