Tilorone-Dihydrochloride Protects against Rift Valley Fever Virus Infection and Disease in the Mouse Model
- PMID: 35056541
- PMCID: PMC8781158
- DOI: 10.3390/microorganisms10010092
Tilorone-Dihydrochloride Protects against Rift Valley Fever Virus Infection and Disease in the Mouse Model
Abstract
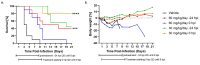
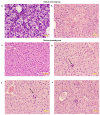
Rift Valley fever (RVF) is a mosquito-borne zoonotic disease endemic to Africa and the Middle East that can affect humans and ruminant livestock. Currently, there are no approved vaccines or therapeutics for the treatment of severe RVF disease in humans. Tilorone-dihydrochloride (Tilorone) is a broad-spectrum antiviral candidate that has previously shown efficacy against a wide range of DNA and RNA viruses, and which is clinically utilized for the treatment of respiratory infections in Russia and other Eastern European countries. Here, we evaluated the antiviral activity of Tilorone against Rift Valley fever virus (RVFV). In vitro, Tilorone inhibited both vaccine (MP-12) and virulent (ZH501) strains of RVFV at low micromolar concentrations. In the mouse model, treatment with Tilorone significantly improved survival outcomes in BALB/c mice challenged with a lethal dose of RVFV ZH501. Treatment with 30 mg/kg/day resulted in 80% survival when administered immediately after infection. In post-exposure prophylaxis, Tilorone resulted in 30% survival at one day after infection when administered at 45 mg/kg/day. These findings demonstrate that Tilorone has potent antiviral efficacy against RVFV infection in vitro and in vivo and supports further development of Tilorone as a potential antiviral therapeutic for treatment of RVFV infection.
Keywords: Bunyavirales; Phenuiviridae; Rift Valley fever virus; Tilorone-dihydrochloride; Viral Hemorrhagic fever; broad-spectrum antiviral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Daubney R., Hudson J.R., Garnham P.C. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. J. Pathol. Bacteriol. 1931;34:545–579. doi: 10.1002/path.1700340418. - DOI
-
- El Mamy A.B., Lô M.M., Thiongane Y., Diop M., Isselmou K., Doumbia B., Baba M.O., El Arbi A.S., Lancelot R., Kane Y., et al. Comprehensive Phylogenetic Reconstructions of Rift Valley Fever Virus: The 2010 Northern Mauritania Outbreak in the Camelus dromedarius Species. Vector-Borne Zoonotic Dis. 2014;14:856–861. doi: 10.1089/vbz.2014.1605. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

