Synthesis and Biological Activity of 3-(Heteroaryl)quinolin-2(1 H)-ones Bis-Heterocycles as Potential Inhibitors of the Protein Folding Machinery Hsp90
- PMID: 35056725
- PMCID: PMC8778022
- DOI: 10.3390/molecules27020412
Synthesis and Biological Activity of 3-(Heteroaryl)quinolin-2(1 H)-ones Bis-Heterocycles as Potential Inhibitors of the Protein Folding Machinery Hsp90
Abstract
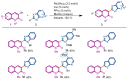
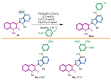
In the context of our SAR study concerning 6BrCaQ analogues as C-terminal Hsp90 inhibitors, we designed and synthesized a novel series of 3-(heteroaryl)quinolin-2(1H), of types 3, 4, and 5, as a novel class of analogues. A Pd-catalyzed Liebeskind-Srogl cross-coupling was developed as a convenient approach for easy access to complex purine architectures. This series of analogues showed a promising biological effect against MDA-MB231 and PC-3 cancer cell lines. This study led to the identification of the best compounds, 3b (IC50 = 28 µM) and 4e, which induce a significant decrease of CDK-1 client protein and stabilize the levels of Hsp90 and Hsp70 without triggering the HSR response.
Keywords: 3-(heteroaryl)quinolin-2(1H)-ones; 6BrCaQ; Hsp90; cytotoxicity; purines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Messaoudi S., Peyrat J.-F., Brion J.-D., Alami M. Recent advances in hsp90 inhibitors as antitumor agents. In: Prudhomme M., editor. Advances in Anti-Cancer Agents in Medicinal Chemistry. 1st ed. Volume 1. Bentham Sciences Publishers; Oak Park, IL, USA: 2013. pp. 107–183. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

