Transition Metal-Free Synthesis of Halobenzo[ b]furans from O-Aryl Carbamates via o-Lithiation Reactions
- PMID: 35056842
- PMCID: PMC8778012
- DOI: 10.3390/molecules27020525
Transition Metal-Free Synthesis of Halobenzo[ b]furans from O-Aryl Carbamates via o-Lithiation Reactions
Abstract
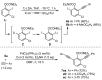
A straightforward and transition metal-free one-pot protocol to synthesize halobenzo[b]furans has been developed employing simple and easily available starting materials such as O-aryl carbamates and alkynylsulfones. The fine-tuning of the different steps involved was key to achieving a successful one-pot procedure. Initially, a directed ortho-lithiation process, which uses the carbamate as the directed metalation group, was crucial in providing access to O-2-alkynylaryl N,N-diethyl carbamates by a direct alkynylation of the o-lithiated carbamate, with arylsulfonylalkynes as electrophilic reagents. Cyclization of the generated o-alkynylaryl carbamates was successfully accomplished through a strategy involving in situ carbamate alkaline hydrolysis under conventional heating or microwave irradiation, coupled with a subsequent heterocyclization step delivering the desired benzo[b]furans. A wide variety of new halobenzo[b]furans has been synthesized and their utility has been demonstrated by their further transformation.
Keywords: alkynylation; benzo[b]furans; carbamates; cyclization; o-lithiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


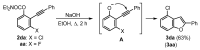

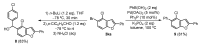
References
-
- Heravi M.M., Zadsirjan V., Hamidi H., Tabar Amiri P.H. Total synthesis of natural products containing benzofuran rings. RSC Adv. 2017;7:24470–24521. doi: 10.1039/C7RA03551A. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

