Pharmacokinetics of Pullulan-Dexamethasone Conjugates in Retinal Drug Delivery
- PMID: 35056906
- PMCID: PMC8779473
- DOI: 10.3390/pharmaceutics14010012
Pharmacokinetics of Pullulan-Dexamethasone Conjugates in Retinal Drug Delivery
Abstract
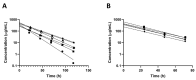
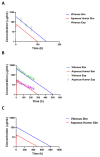
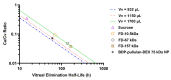
The treatment of retinal diseases by intravitreal injections requires frequent administration unless drug delivery systems with long retention and controlled release are used. In this work, we focused on pullulan (≈67 kDa) conjugates of dexamethasone as therapeutic systems for intravitreal administration. The pullulan-dexamethasone conjugates self-assemble into negatively charged nanoparticles (average size 326 ± 29 nm). Intravitreal injections of pullulan and pullulan-dexamethasone were safe in mouse, rat and rabbit eyes. Fluorescently labeled pullulan particles showed prolonged retention in the vitreous and they were almost completely eliminated via aqueous humor outflow. Pullulan conjugates also distributed to the retina via Müller glial cells when tested in ex vivo retina explants and in vivo. Pharmacokinetic simulations showed that pullulan-dexamethasone conjugates may release free and active dexamethasone in the vitreous humor for over 16 days, even though a large fraction of dexamethasone may be eliminated from the eye as bound pullulan-dexamethasone. We conclude that pullulan based drug conjugates are promising intravitreal drug delivery systems as they may reduce injection frequency and deliver drugs into the retinal cells.
Keywords: conjugate; dexamethasone; ocular fluorophotometry; optical coherence tomography; pharmacokinetics; pullulan; retinal drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Cohen S.Y., Mimoun G., Oubraham H., Zourdani A., Malbrel C., Queré S., Schneider V., Group L.S. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: The LUMIERE study. Retina. 2013;33:474–481. doi: 10.1097/IAE.0b013e31827b6324. - DOI - PubMed
-
- Holz F.G., Tadayoni R., Beatty S., Berger A., Cereda M.G., Cortez R., Hoyng C.B., Hykin P., Staurenghi G., Heldner S. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br. J. Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

