A Triple Combination of Targeting Ligands Increases the Penetration of Nanoparticles across a Blood-Brain Barrier Culture Model
- PMID: 35056983
- PMCID: PMC8778049
- DOI: 10.3390/pharmaceutics14010086
A Triple Combination of Targeting Ligands Increases the Penetration of Nanoparticles across a Blood-Brain Barrier Culture Model
Abstract
Nanosized drug delivery systems targeting transporters of the blood-brain barrier (BBB) are promising carriers to enhance the penetration of therapeutics into the brain. The expression of solute carriers (SLC) is high and shows a specific pattern at the BBB. Here we show that targeting ligands ascorbic acid, leucine and glutathione on nanoparticles elevated the uptake of albumin cargo in cultured primary rat brain endothelial cells. Moreover, we demonstrated the ability of the triple-targeted nanovesicles to deliver their cargo into midbrain organoids after crossing the BBB model. The cellular uptake was temperature- and energy-dependent based on metabolic inhibition. The process was decreased by filipin and cytochalasin D, indicating that the cellular uptake of nanoparticles was partially mediated by endocytosis. The uptake of the cargo encapsulated in triple-targeted nanoparticles increased after modification of the negative zeta potential of endothelial cells by treatment with a cationic lipid or after cleaving the glycocalyx with an enzyme. We revealed that targeted nanoparticles elevated plasma membrane fluidity, indicating the fusion of nanovesicles with endothelial cell membranes. Our data indicate that labeling nanoparticles with three different ligands of multiple transporters of brain endothelial cells can promote the transfer and delivery of molecules across the BBB.
Keywords: ascorbic acid; blood-brain barrier; brain endothelial cell; brain organoid; drug targeting; glutathione; leucine; nanoparticle; solute carrier; triple targeting ligand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




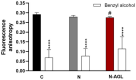
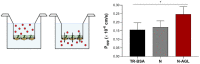
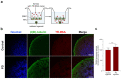
References
Grants and funding
- NNE-29617/National Research, Development and Innovation Office
- GINOP-2.2.1-15-2016-00007/National Research, Development and Innovation Office
- GINOP-2.3.2-15-216-00060/National Research, Development and Innovation Office
- Premium-2019-469/Hungarian Academy of Sciences
- PD-138930/National Research, Development and Innovation Office
LinkOut - more resources
Full Text Sources

