SonoVue® vs. Sonazoid™ vs. Optison™: Which Bubble Is Best for Low-Intensity Sonoporation of Pancreatic Ductal Adenocarcinoma?
- PMID: 35056994
- PMCID: PMC8777813
- DOI: 10.3390/pharmaceutics14010098
SonoVue® vs. Sonazoid™ vs. Optison™: Which Bubble Is Best for Low-Intensity Sonoporation of Pancreatic Ductal Adenocarcinoma?
Abstract
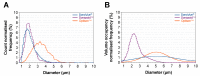
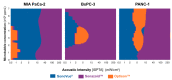
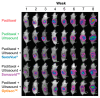
The use of ultrasound and microbubbles to enhance therapeutic efficacy (sonoporation) has shown great promise in cancer therapy from in vitro to ongoing clinical studies. The fastest bench-to-bedside translation involves the use of ultrasound contrast agents (microbubbles) and clinical diagnostic scanners. Despite substantial research in this field, it is currently not known which of these microbubbles result in the greatest enhancement of therapy within the applied conditions. Three microbubble formulations-SonoVue®, Sonazoid™, and Optison™-were physiochemically and acoustically characterized. The microbubble response to the ultrasound pulses used in vivo was simulated via a Rayleigh-Plesset type equation. The three formulations were compared in vitro for permeabilization efficacy in three different pancreatic cancer cell lines, and in vivo, using an orthotopic pancreatic cancer (PDAC) murine model. The mice were treated using one of the three formulations exposed to ultrasound from a GE Logiq E9 and C1-5 ultrasound transducer. Characterisation of the microbubbles showed a rapid degradation in concentration, shape, and/or size for both SonoVue® and Optison™ within 30 min of reconstitution/opening. Sonazoid™ showed no degradation after 1 h. Attenuation measurements indicated that SonoVue® was the softest bubble followed by Sonazoid™ then Optison™. Sonazoid™ emitted nonlinear ultrasound at the lowest MIs followed by Optison™, then SonoVue®. Simulations indicated that SonoVue® would be the most effective bubble using the evaluated ultrasound conditions. This was verified in the pre-clinical PDAC model demonstrated by improved survival and largest tumor growth inhibition. In vitro results indicated that the best microbubble formulation depends on the ultrasound parameters and concentration used, with SonoVue® being best at lower intensities and Sonazoid™ at higher intensities.
Keywords: microbubbles; pancreatic cancer; sonoporation; targeted drug delivery; ultrasound; ultrasound-enhanced therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources

