Elovanoids Counteract Inflammatory Signaling, Autophagy, Endoplasmic Reticulum Stress, and Senescence Gene Programming in Human Nasal Epithelial Cells Exposed to Allergens
- PMID: 35057008
- PMCID: PMC8778361
- DOI: 10.3390/pharmaceutics14010113
Elovanoids Counteract Inflammatory Signaling, Autophagy, Endoplasmic Reticulum Stress, and Senescence Gene Programming in Human Nasal Epithelial Cells Exposed to Allergens
Abstract
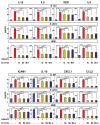
To contribute to further understanding the cellular and molecular complexities of inflammatory-immune responses in allergic disorders, we have tested the pro-homeostatic elovanoids (ELV) in human nasal epithelial cells (HNEpC) in culture challenged by several allergens. ELV are novel bioactive lipid mediators synthesized from the omega-3 very-long-chain polyunsaturated fatty acids (VLC-PUFA,n-3). We ask if: (a) several critical signaling events that sustain the integrity of the human nasal epithelium and other organ barriers are perturbed by house dust mites (HDM) and other allergens, and (b) if ELV would participate in beneficially modulating these events. HDM is a prevalent indoor allergen that frequently causes allergic respiratory diseases, including allergic rhinitis and allergic asthma, in HDM-sensitized individuals. Our study used HNEpC as an in vitro model to study the effects of ELV in counteracting HDM sensitization resulting in inflammation, endoplasmic reticulum (ER) stress, autophagy, and senescence. HNEpC were challenged with the following allergy inducers: LPS, poly(I:C), or Dermatophagoides farinae plus Dermatophagoides pteronyssinus extract (HDM) (30 µg/mL), with either phosphate-buffered saline (PBS) (vehicle) or ELVN-34 (500 nM). Results show that ELVN-34 promotes cell viability and reduces cytotoxicity upon HDM sensitization of HNEpC. This lipid mediator remarkably reduces the abundance of pro-inflammatory cytokines and chemokines IL-1β, IL-8, VEGF, IL-6, CXCL1, CCL2, and cell adhesion molecule ICAM1 and restores the levels of the pleiotropic anti-inflammatory IL-10. ELVN-34 also lessens the expression of senescence gene programming as well as of gene transcription engaged in pro-inflammatory responses. Our data also uncovered that HDM triggered the expression of key genes that drive autophagy, unfolded protein response (UPR), and matrix metalloproteinases (MMP). ELVN-34 has been shown to counteract these effects effectively. Together, our data reveal a novel, pro-homeostatic, cell-protective lipid-signaling mechanism in HNEpC as potential therapeutic targets for allergies.
Keywords: allergy; anti-inflammatory; elovanoids; house dust mite; inflammation; lipid mediators.
Conflict of interest statement
The authors declare no conflict of interest. Diater Laboratorio had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures





References
-
- Foray A.-P., Dietrich C., Pecquet C., Machavoine F., Chatenoud L., Leite-de-Moraes M. IL-4 and IL-17 Are Required for House Dust Mite-Driven Airway Hyperresponsiveness in Autoimmune Diabetes-Prone Non-Obese Diabetic Mice. Front. Immunol. 2021;11:595003. doi: 10.3389/fimmu.2020.595003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

