Enzyme-Responsive Amphiphilic Peptide Nanoparticles for Biocompatible and Efficient Drug Delivery
- PMID: 35057039
- PMCID: PMC8779831
- DOI: 10.3390/pharmaceutics14010143
Enzyme-Responsive Amphiphilic Peptide Nanoparticles for Biocompatible and Efficient Drug Delivery
Abstract
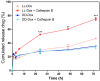
Self-assembled peptide nanostructures recently have gained much attention as drug delivery systems. As biomolecules, peptides have enhanced biocompatibility and biodegradability compared to polymer-based carriers. We introduce a peptide nanoparticle system containing arginine, histidine, and an enzyme-responsive core of repeating GLFG oligopeptides. GLFG oligopeptides exhibit specific sensitivity towards the enzyme cathepsin B that helps effective controlled release of cargo molecules in the cytoplasm. Arginine can induce cell penetration, and histidine facilitates lysosomal escape by its buffering capacity. Herein, we propose an enzyme-responsive amphiphilic peptide delivery system (Arg-His-(Gly-Phe-Lue-Gly)3, RH-(GFLG)3). The self-assembled RH-(GFLG)3 globular nanoparticle structure exhibited a positive charge and formulation stability for 35 days. Nile Red-tagged RH-(GFLG)3 nanoparticles showed good cellular uptake compared to the non-enzyme-responsive control groups with d-form peptides (LD (LRH-D(GFLG)3), DL (DRH-L(GFLG)3), and DD (DRH-D(GFLG)3). The RH-(GFLG)3 nanoparticles showed negligible cytotoxicity in HeLa cells and human RBCs. To determine the drug delivery efficacy, we introduced the anticancer drug doxorubicin (Dox) in the RH-(GFLG)3 nanoparticle system. LL-Dox exhibited formulation stability, maintaining the physical properties of the nanostructure, as well as a robust anticancer effect in HeLa cells compared to DD-Dox. These results indicate that the enzyme-sensitive RH-(GFLG)3 peptide nanoparticles are promising candidates as drug delivery carriers for biomedical applications.
Keywords: cytotoxicity; doxorubicin; drug delivery systems; peptide nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

