A Combined Atomic and Molecular Probe Characterization of Aromatic Hydrocarbons via PALS and ESR: Methylbenzene
- PMID: 35057182
- PMCID: PMC8777640
- DOI: 10.3390/ma15020462
A Combined Atomic and Molecular Probe Characterization of Aromatic Hydrocarbons via PALS and ESR: Methylbenzene
Abstract
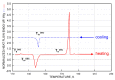
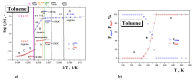
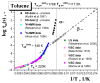
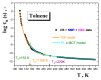
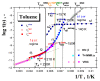
A combined study of one of the simplest aromatic hydrocarbons, i.e., methylbenzene (toluene) (TOL), via the annihilation of an ortho-positronium (o-Ps) probe via positron annihilation lifetime spectroscopy (PALS) and the rotation dynamics of nitroxide spin probe 2,2,6,6-tetramethyl-piperidinyl-1-oxy (TEMPO) using electron spin resonance (ESR) over a wide temperature range, 10-300 K, is reported. The o-Ps lifetime, τ3, and the relative o-Ps intensity, I3, as a function of temperature exhibit changes defining several characteristic PALS temperatures in the slowly and rapidly cooled samples. Similarly, the spectral parameter of TEMPO mobility in TOL, 2Azz', and its correlation time, τc, reveal several effects at a set of the characteristic ESR temperatures, which were determined and compared with the PALS results. Finally, the physical origins of the changes in free volume expansion and spin probe mobility are revealed. They are reflected in a series of the mutual coincidences between the characteristic PALS and ESR temperatures and appropriate complementary thermodynamic and dynamic techniques.
Keywords: ESR; PALS; free volume; methylbenzene or toluene; positronium; relaxation dynamics; spin probe dynamics; thermodynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Švajdlenková H., Šauša O., Iskrová-Miklošovicová M., Majerník V., Krištiak J., Bartoš J. On the relationships between guest molecular dynamics and free volume in a series of small molecular and polymer glass-formers. Chem. Phys. Lett. 2012;539:39–44. doi: 10.1016/j.cplett.2012.05.013. - DOI
-
- Švajdlenková H., Iskrová M., Šauša O., Dlubek G., Krištiak J., Bartoš J. The Spin Probe Dynamics and the Free Volume in a Series of Amorphous Polymer Glass-Formers. Macromol. Symp. 2011;305:108–115. doi: 10.1002/masy.201000103. - DOI
-
- Švajdlenková H., Bartoš J. Spin probe mobility in relation to free volume and relaxation dynamics of glass-formers: A series of spin probes in poly (isobutylene) J. Polym. Sci. B Polym. Phys. 2009;47:1058–1068. doi: 10.1002/polb.21710. - DOI
-
- Bartoš J., Švajdlenková H., Šauša O., Lukešová M., Ehlers D., Michl M., Lunkenheimer P., Loidl A. Molecular probe dynamics and free volume in organic glass-formers and their relationships to structural relaxation: 1-propanol. J. Phys.-Cond. Matter. 2016;28:015101. doi: 10.1088/0953-8984/28/1/015101. - DOI - PubMed
-
- Bartoš J., Švajdlenková H. On the mutual relationships between spin probe mobility, free volume and relaxation dynamics in organic glass-formers: Glycerol. Chem. Phys. 2017;670:58–63. doi: 10.1016/j.cplett.2016.12.064. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

